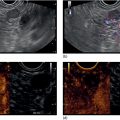
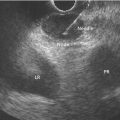
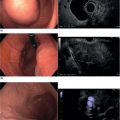
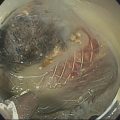
Brenna Casey1 and Kumar Krishnan2 1 Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA 2 Interventional Endoscopy, Harvard Medical School, Massachusetts General Hospital, Boston, MA, USA German anatomist Abraham Vater (1684–1751) first described a tubular structure which consists of the confluence of the distal common bile duct and main pancreatic duct in the second portion of the duodenum. This convergence is now known as the ampulla of Vater. In about 85% of individuals the pancreatic duct and common bile duct join in the ampulla and form a distal common channel. The normal ampulla starts about 2 mm outside the duodenal wall and penetrates the muscularis propria somewhat more distally, forming an intraduodenal segment 9–25 mm in length. Tumors of the ampulla of Vater originate from the pancreaticobiliary–duodenal junction, limited by the sphincter of Oddi. Ampullary tumors may present with obstructive jaundice, duodenal obstruction or bleeding, or they may be discovered as an incidental finding at the time of upper gastrointestinal endoscopy or barium radiography for unrelated symptoms. While the presenting symptoms may be very similar to those of pancreatic carcinoma, ampullary malignancies have a much better prognosis. Their evolution appears to follow the adenoma–carcinoma sequence and they are usually discovered at an earlier stage than pancreatic tumors. Ampullary tumors are generally undetectable on transabdominal ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT) due to their small size. Endoscopically they present as polypoid exaggerations of the ampulla. Endoscopic ultrasonography (EUS) provides high‐resolution images and permits positioning of the ultrasound transducer in close approximation to the tumor in the duodenum. Endoscopic ultrasonography is specifically useful for T and N staging, which is important in determining the method of resection and ultimately the prognosis of patients with ampullary carcinomas (Figures 19.1 and 19.2). Endoscopic ultrasound imaging of ampullary neoplasia is achieved by positioning the echoendoscope directly adjacent to the ampulla itself. Acoustic coupling is aided by a synergy created by positioning the water‐filled balloon adjacent to the lesion while water is slowly (to avoid formation of bubbles which will obscure the detail of the images) instilled into the lumen of the duodenum (Figure 19.3). Particularly in small tumors, it is essential to avoid overfilling the balloon, as the ampullary lesion may be easily compressed by the balloon resulting in impaired visualization or in some cases overlooking the lesion completely. On the other hand, overfilling of the balloon can compress the tumor and underlying wall layers together, leading to artifact and misinterpretation resulting in overstaging. The patient is usually in the left lateral position, but in some cases a supine position or right lateral decubitus may improve imaging success, especially when the lesions are very small making a stable imaging position challenging. Tumors of the ampulla present as localized wall thickening of the duodenum at the level of the ampulla. The dilated distal common bile duct can be traced into the tumor and neoplastic growth may be seen in the dilated lumen. Smaller tumors are usually echo‐homogeneous and echo‐poor; inhomogeneous internal echoes occur more frequently with increasing tumor size. An adenoma of the ampulla does not involve the duodenal wall layers whereas a more advanced carcinoma infiltrates the duodenal wall layers or the pancreas. Differentiation of a T1 carcinoma (limited to the ampulla) from an adenoma is not possible by means of EUS. Tumors of the periampullary regions may be either exophytic polypoid or hypoechoic mass lesions in the area of the ampulla that may be indistinguishable from distal pancreatic carcinomas (Video 19.1). Although biopsies of the polypoid lesions may only show adenomatous tissue, frequently a small focus of malignancy is present. Endoscopic ultrasound is not capable of differentiating a small focus of malignancy within an otherwise benign adenoma. Only in the case of deep infiltrative growth can the endosonographer discern the presence of a malignancy. A recent sphincterotomy and the resulting inflammatory response to the thermal injury may create artifacts which mimic pseudopodia signifying inifiltrative growth. Endoscopic ultrasound cannot replace histology and should be used to stage ampullary lesions of known histological status. For villous adenomas of the ampulla of Vater, extension of the villous tissue into the common bile duct can occasionally be seen. Figure 19.1 The linear array echoendoscope is in the duodenum directly adjacent to the ampulla. There is a large, exophytic, hypoechoic ampullary mass infiltrating the duodenal wall and filling the lumen. The lesion does not penetrate through the muscularis propria (MP, arrowhead). Figure 19.2 A hypoechoic mass is seen here arising from the ampulla. The malignancy is invading through the duodenal wall, surrounding the bile duct and into the pancreas, which makes this lesion a stage T3 ampullary cancer. Staging according to the TNM system is as follows. Metastatic lymph nodes are classified using the same uniformly accepted criteria (size or echo pattern). Accurate local staging is important in centers where endoscopic ampullectomy is performed. However, in most cases local staging does not alter management, as the lesion is removed during a pancreaticoduodenectomy (Whipple procedure). Endoscopic ultrasound detects small ampullary neoplasms when other imaging modalities reveal biliary or pancreatic ductal dilatation in the absence of a detectable mass. It also permits identification of infiltration through the duodenal muscularis propria and invasion into the pancreatic parenchyma. The accuracy of EUS in assessing the depth of tumor invasion and in detecting local lymph node metastases has been reported to be better than 80% for T stage and 70% for N stage. Overstaging in stage T1 occurs in up to one‐third of cases and depends greatly on operator technique. However, infiltration into the pancreas, which is associated with a poorer prognosis, is detected in nearly 90% of cases using EUS. From a surgical standpoint, carcinoma of the ampulla can nearly always be resected unless it infiltrates the peripancreatic vessels, in which case a pancreatic primary infiltrating the ampulla should be considered. Other imaging modalities such at CT, abdominal ultrasound, and MRI, while not useful for T‐staging of ampullary carcinoma, are necessary adjuncts for complete N‐staging and M‐staging prior to surgery and/or initiation of chemotherapy/radiation therapy. Intraductal ultrasound probes are useful for assessing tumor extension into the distal common bile duct in villous or papillary‐type lesions prior to consideration for endoscopic resection, particularly in those cases in which the limits of the lesion are not clear on cholangiography. Care must be taken to avoid excessive pressure with the duodenoscope elevator on the intraductal ultrasound (IDUS) probe, as well as attention paid to the acute bend that is formed when the probe leaves the working channel of the duodenoscope and enters the bile duct, which may lead to poor imaging quality as well as probe breakage. For elderly patients with locally advanced disease who are not good surgical candidates, palliation of obstructive symptoms may be provided by debulking of the lesion, endoscopic sphincterotomy, and biliary stent placement.
19
Duodenal and Ampullary Neoplasia


TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
T1
Tumor 1 cm or less (tumor limited to ampulla of Vater for ampullary gangliocytic paraganglioma)
T2
Tumor >1 cm
T3
Tumor invades pancreas or retroperitoneum or into non‐peritonealized tissues
T4
Tumor invades visceral peritoneum (serosa) or invades other organs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree