The normal breast tissue undergoes marked alterations in size, shape, and function in response to the onset of puberty, pregnancy, lactation, and menopause. Both endogenous (early menarche, older age at first pregnancy, and nulliparity) and exogenous (use of hormonal replacement therapy) hormonal exposures are associated with these physiological states and were identified as risk factors for developing breast cancer. Therefore recognizing the unique imaging manifestations in each physiological state is of both diagnostic and prognostic significance.
Menstrual Cycle
Upon the onset of menarche, the breast tissue fluctuates in response to the hormonal regulation throughout the menstrual cycle. Several distinct microscopic characteristics have been defined for the follicular and luteal phases, which vary in the vascular and histological features of the epithelial and stromal components. Dense and cellular stroma without active mammary glandular secretion are highlights of the follicular phase, whereas the luteal phase is characterized by stromal edema, basal cell vacuolization, duct distension, venous congestion, and active glandular secretion. These histological differences may be reflected in imaging studies, accounting for the periodic changes of structural and perfusion measurements. Indeed, variations in fibroglandular tissue amount were around 7% in a quantitative, longitudinal T2-weighted study during the cycle. Moreover, the breast’s vascular network fluctuates, as an upsurge in progesterone blood levels lead to periodic vascular dilation and consequently, variations in background parenchymal enhancement (BPE) appear on dynamic contrast-enhanced magnetic resonance imaging (DCE MRI) along the cycle. This less commonly occurs in the second week of menstruation, which is therefore the recommended timing for scan scheduling.
Unlike perfusion studies, diffusion MRI of the breast is less likely to be affected by mild physiological vascular variations. In fact, the intravoxel incoherent motion (IVIM) phenomenon of the perfusion-related (see Chapter 1 ), fast-decaying component on the biexponent model is hardly apparent in the normal breast, as opposed to the pronounced vascular supply of locally advanced invasive carcinoma. Several studies have reported on the effects of the menstrual cycle on diffusion MRI and apparent diffusion coefficient (ADC) values. Partridge and colleagues reported a nonstatistically significant 5% variation among four weekly ADC measurements of healthy, premenopausal volunteers scanned throughout one menstrual cycle. O’Flynn and colleagues and Al Rashidi and colleagues reported similar breast ADC values in the proliferative and secretory phases, whereas Clendenen and colleagues found up to a 14% increase in the ADC luteal phase in comparison with the follicular phase of the cycle. Furthermore, Wiederer and colleagues concluded that breast diffusion tensor imaging (DTI; see Chapter 1, Chapter 9 ) parameters do not depend on the menstrual cycle, which is in agreement with another report on stable parametric measurements of the DTI properties during the cycle. This study reported a nonstatistically significant intraindividual coefficient of variance between 1% and 2% for the diffusion coefficients and 5% for fractional anisotropy (FA). All in all, the studies varied in design, most notably in the sample size, scanning protocol, number of scans, and means of image analysis. Despite the apparent disagreement between some of these studies, from a clinical perspective, all the studies support the conclusion that the diffusivity variations along the cycle are negligible, in comparison with the more drastic diffusivity reduction, associated with malignancy. From a practical radiological standpoint, unlike the effect of the different phases of the menstrual cycle on breast cancer detectability on screening mammography and DCE MRI, the menstrual cycle significantly affects neither tumor detectability nor ADC values of the tumor and normal fibroglandular tissue among breast cancer patients. Thus the utility of diffusion MRI of the breast is not dependent on menstrual cycle effects.
Pregnancy and Lactation
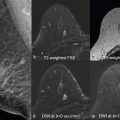
During pregnancy, the breast experiences unique physiological and morphological transformations until it reaches its eventual functional competence during lactation. Ultimately, the mammary gland becomes significantly larger, and the relative composition of its three fundamental elements—glandular tissue, stroma, and adipose tissue—completely changes. There is marked glandular tissue (lobules and ducts) proliferation, resulting in a prominent and distended ductal tree at the expense of stromal involution and reduced fat distribution. In order to fulfill its functional role of synthesizing and secreting milk, the breast undergoes two stages of lactogenesis, which is regulated by key hormones. Lactogenesis I stage (secretory initiation) begins during the second trimester of pregnancy. However, the actual secretion of milk is stimulated only within days of the postpartum period, in the so-called lactogenesis II stage (secretory activation). Triggered by a rapid drop in progesterone, the milk’s volume and composition initially presents as colostrum enriched with protein and electrolytes; however, after several days of breastfeeding it changes into a mature, stable mother’s milk, with increased lipid content and decreased protein, sodium, and chloride concentrations. An illustration of the lactating breast microstructure and representative DTI mean diffusivity map is shown in Fig. 7.1 , which highlights the prominent ductal tree during pregnancy and lactation.
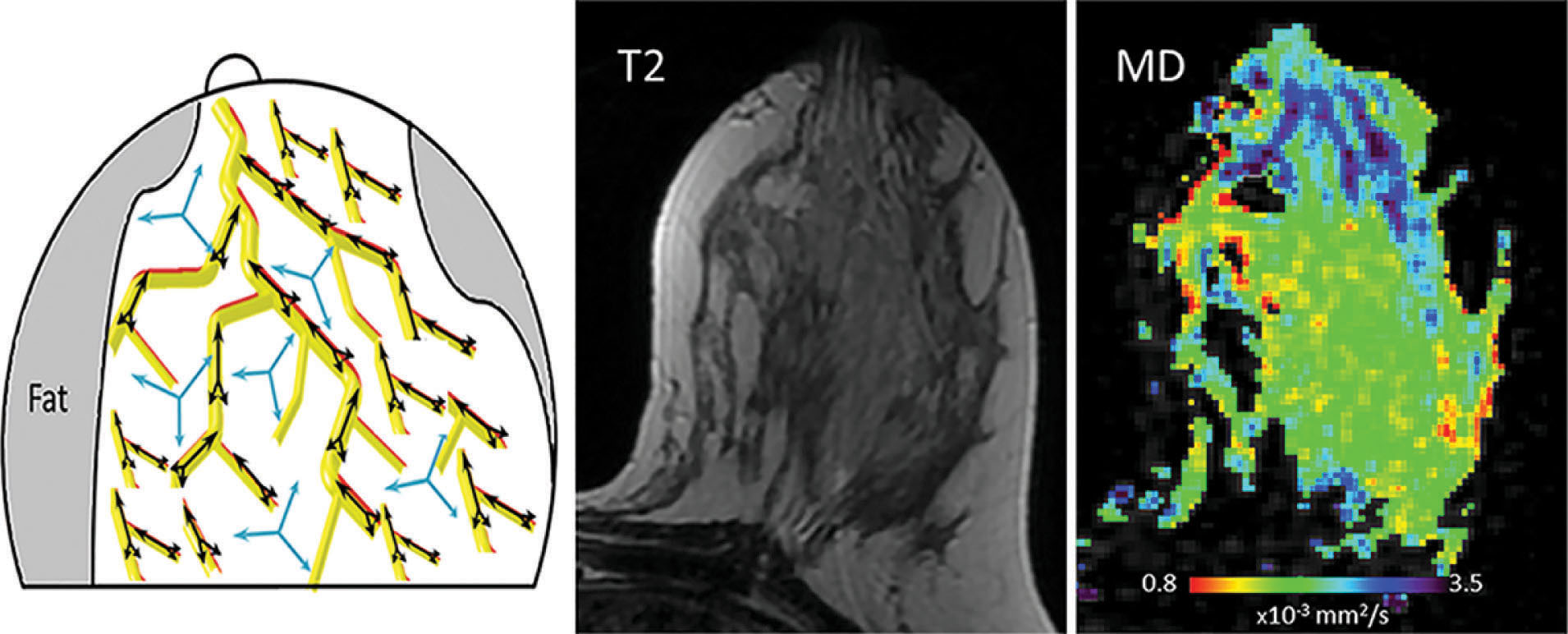
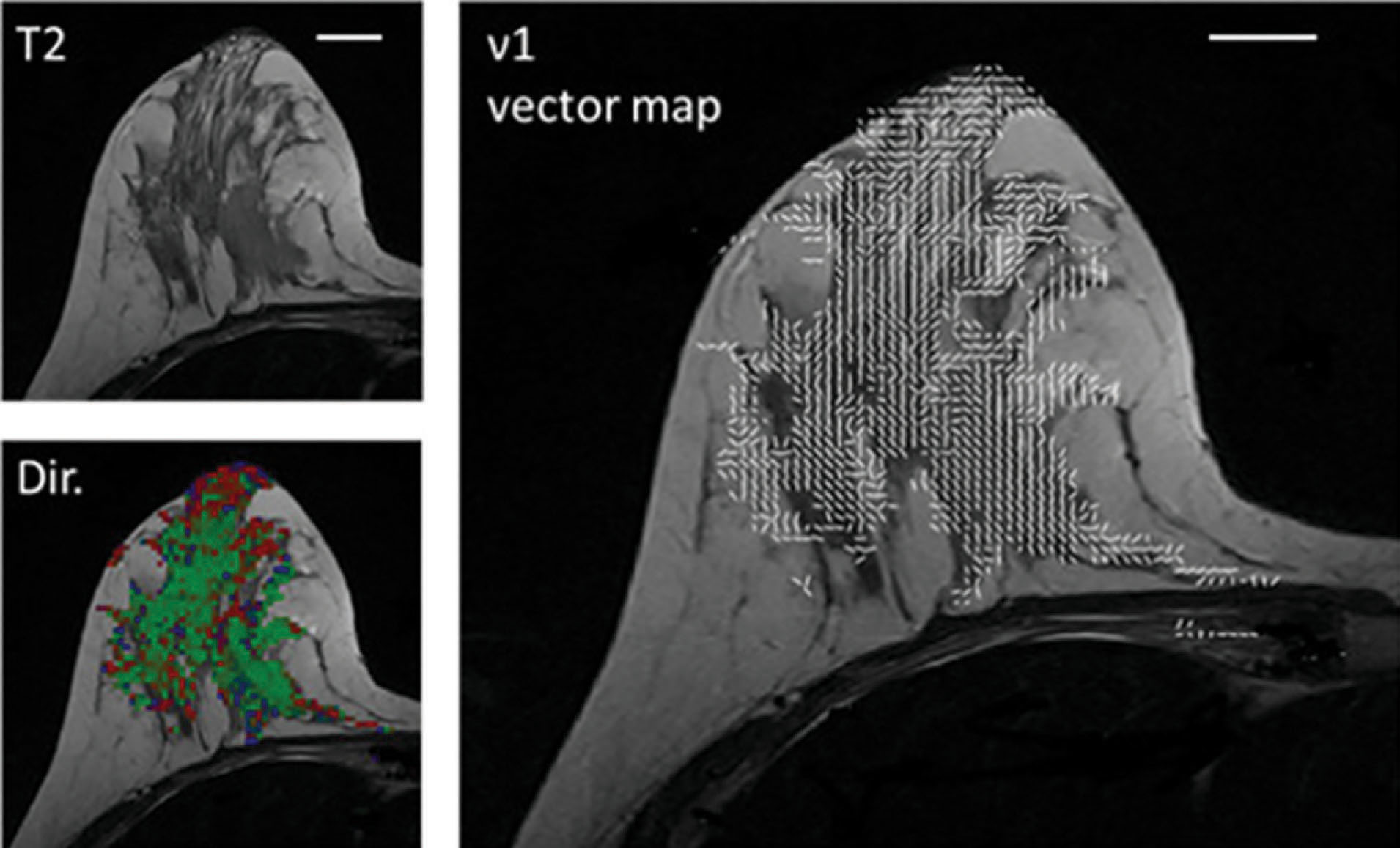
The unique features of the breast during pregnancy and lactation have been characterized by several diffusion MRI studies. The diffusion tensor properties of healthy pregnant examinees were found to resemble the ones of the premenopausal, nonpregnant population, with relatively high values of diffusivity, which is typical for dense breasts. On the other hand, during lactation, three independent groups reported a relative decrease in the lactating parenchyma diffusivity (compared with nonlactating controls) using three different models: the diffusion-weighted imaging (DWI), IVIM, and DTI protocols. The marked decrease in diffusivity between pregnancy and lactation could be attributed to the viscous nature of fat-rich milk, which causes slow diffusivity. Moreover, the lactating breast has been characterized by increased perfusion fraction (f) measured with the IVIM approach, which is anticipated in the view of the breast’s increase in vascularity, which serves to meet the high metabolic demand of lactation. Interestingly, when comparing the intraindividual DWI IVIM properties of lactating volunteers on scans before and after milk pumping/breastfeeding, it demonstrates that the diffusivity is significantly decreased postbreastfeeding. A possible clinical implication of this finding could be the recommendation to perform breast diffusion MRI scans before breastfeeding rather than the recommendation to pump milk before mammography, though a comparative study is needed to confirm or deny this hypothesis. A further microstructural characterization of the lactating breast was afforded by DTI studies, which reported reduced anisotropy, probably due to the increase in the physiological diameter of the ducts. Finally, diffusion tensor MRI has also enabled probing of the underlying ductal architecture of the lactating breast, exemplified by the diffusivity with direction predominance toward the nipple and noticeable duct-like, linear appearing vectors of the first eigenvalue, as shown in Fig. 7.2 .
Involution
Upon weaning, the breast returns to its normal preconception, physiological condition in three gradual, overlapping steps. The mammary gland undergoes dramatic involution characterized by extensive epithelial apoptosis, regrowth of the mammary adipose tissue, and tissue remodeling, which resembles the inflammatory state. The size and secretory activity of the human mammary gland decline slowly as the infant begins to eat other foods, until ultimately the composition of the breast evolves, inducing withdrawal of the prominent ductal tree, which is typical of lactation and replacement with stromal and fat tissues. Interestingly, after lactation and involution, breast vascularity is reset at a lower level compared with baseline.
Quantitative characterization of the diffusivity and microstructural changes of the mammary gland throughout involution was represented by a recent intraindividual longitudinal study. In this work, a cohort of healthy lactating volunteers were scanned twice, at the time of lactation and postweaning, using anatomical T2-weighted and DTI protocols. Anatomical comparison revealed a 42% and 41% reduction in the breast size and fibroglandular tissue percentage, respectively. Furthermore, a significant decrease in the radial diffusivity coefficients and an increase in anisotropy indices were noted postweaning, whereas axial diffusivity remained unchanged. The authors attributed these findings to the expected decrease in the mammary ductal diameter in the presence of a transient, milk-like medium. In another study, diffusion and IVIM parameters were compared between cohorts of lactating and postweaning healthy volunteers. Findings revealed that ADC values tended to be higher in postweaning breasts compared with the lactation period, with significantly lower kurtosis coefficient (K). Perfusion fraction (f) significantly decreased in postweaning breasts, possibly reflecting the decreased vascularity postweaning.
Pregnancy-Associated Breast Cancer
Pregnancy-associated breast cancer (PABC) is commonly defined as breast cancer diagnosed during either pregnancy, lactation or in the first year postpartum (PABC). PABC accounts for about 1% of all breast cancers; however, with the trend toward delayed parenthood, its incidence is on the rise. During pregnancy and lactation, the breast is characterized with palpable nodularity, firmness, and increased mammographic density, making both clinical and radiological examination more challenging. As a consequence, PABC is often a delayed diagnosis and carries a poor prognosis. It is detected only after clinical symptoms present, usually in the form of a large palpable mass. Interestingly, PABC’s prognosis does not differ from that of nonpregnant women when adjusted for stage and age. This suggests that the delay in diagnosis, rather than the unique biological environment with overexpression of vascular, hormonal, and growth-factor mediators, is the main cause for the dismal prognosis. This demonstrates the unmet necessity for developing and using better imaging modalities for achieving early diagnosis.
During pregnancy, breast DCE MRI is contraindicated due to safety concerns related to the use of gadolinium-based contrast agents, which are known to cross the placenta. Breast DCE MRI studies during pregnancy were limited to scans that were conducted immediately before planned abortion. Gadolinium is considered safe during lactation, yet DCE utility is hampered due to the marked BPE that is characteristic of lactation. Consequently, annual breast MRI screening examinations for high-risk patients and MRI diagnostic workups are not performed during pregnancy and are even further postponed until weaning. In the attempt to use unenhanced breast DWI as a stand-alone screening modality, using diffusion MRI to achieve an early diagnosis of PABC appears to be an ideal case-study application.
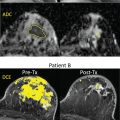
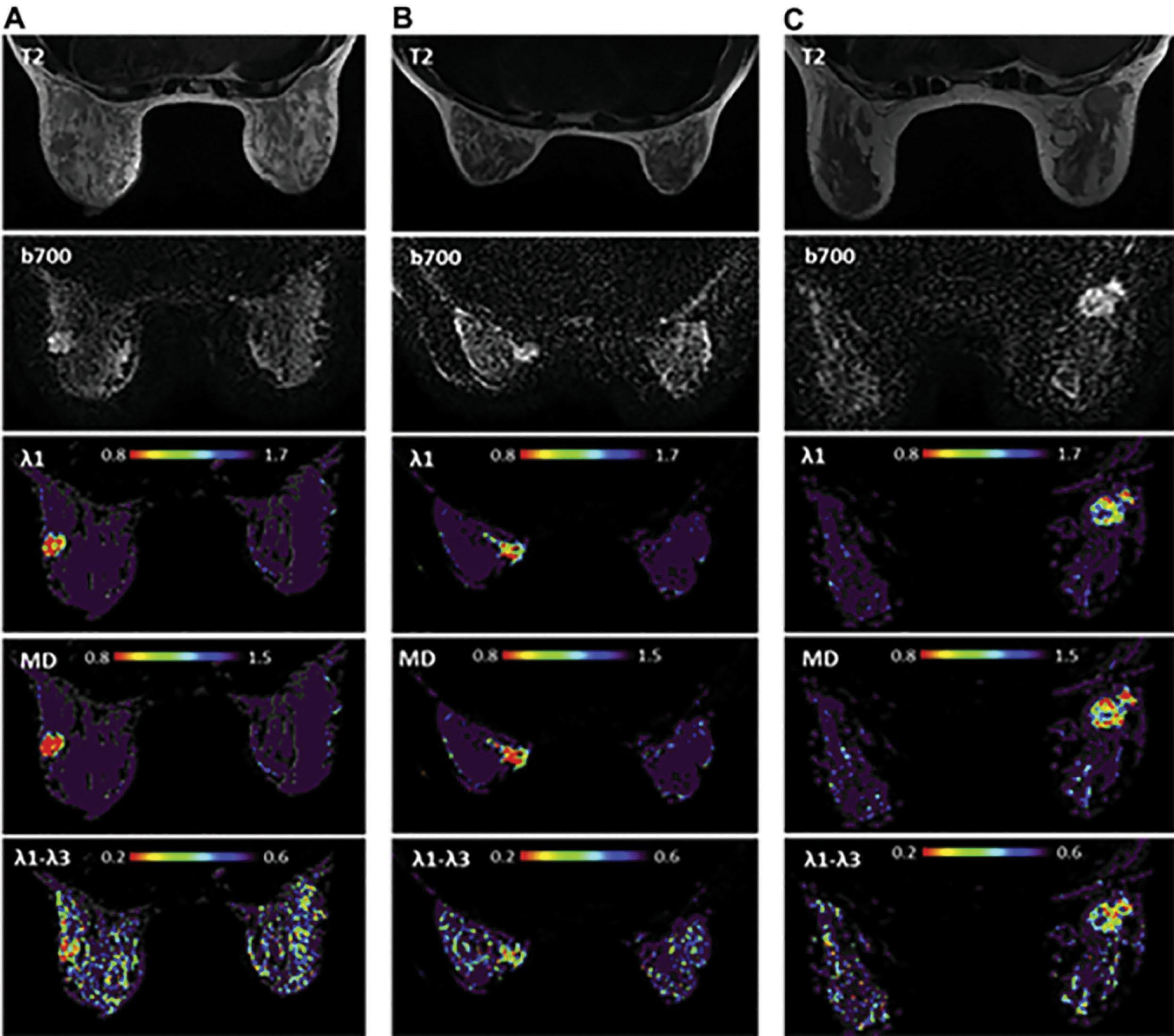
Indeed, the use of breast DTI among pregnant patients at high risk or with newly diagnosed PABC was recently reported. This study, with a relatively small cohort, demonstrated the feasibility and tolerability of breast MRI examination in the prone position among pregnant patients, including in the third trimester. Moreover, DTI parametric maps were found to be useful in detecting the tumor location and extent, albeit, the inability of the method to detect 7-mm tumor foci, as anticipated by the current technical drawbacks of this modality. Representative cases of unenhanced DTI of PABC are shown in Fig. 7.3 , highlighting the evident diagnostic capabilities of this approach.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree