, Jean-Philippe Bault2, Bernard Benoit3 and Gérard Couly4
(1)
Center of women and fetal imaging, Créteil, France
(2)
Center of fetal imaging Ambroise Paré, Les Mureaux, France
(3)
Princess Grace Hospital, Monaco, France
(4)
Department of maxillo-facial surgery, Necker Hospital, Paris, France
4.1 How to Define Objective Facial Dysmorphism
The study of fetal face anomalies using precise, reproducible, anthropometric criteria helps avoid the too-frequent subjectivity found in an approach that is not truly quantified. The discovery of dysmorphism and its characterization is one way that is frequently used to uncover various pathological fetal syndromes.
4.2 Tools for This Study
They were described in Chap. 1 and consist of:
Volume ultrasound images that enable:
Capturing rigorously strict views using multiple plane views and their orthogonality principle.
Demonstrative imagery with surface rendering modes
An evaluation of the bone structures with the “maximum” mode.
Thus, the study of the sagittal view, especially with a study of the facial angles, is a key moment in the study of the fetal profile, which is very often altered in cases of facial dysmorphism.
The axial view will contribute key information regarding the shape and size of the maxillary bones and particularly the mandible, and will, among other things, enable a precise measurement of the inter-orbital diameter.
The coronal view will, as shown in other chapters of this book, be an important tool to evaluate labioalveolar-palate defects.
A panel of measurements covers the various facial parameters.
For a clear presentation, we decided to subdivide the most common dysmorphic pathologies that impact the face into five categories:
Major chromosomal anomalies
Pathologies impacting primarily the upper face
Pathologies impacting primarily the mid-face
Pathologies impacting primarily the lower face
Unclassifiable pathologies
4.3 Dysmorphism of Major Chromosomal Anomalies
Screening for the major chromosomal anomalies currently depends on quality screening during the first trimester. In this chapter, we will identify the principal characteristics of the dysmorphism related to the major chromosomal anomalies.
4.3.1 Trisomy 21
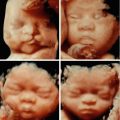
There is frequently a delay in the ossification of the nose bones (Figs. 4.1 and 4.2), the forehead could be domed, sometimes with a prefrontal edema (Fig. 4.3), the features could appear flat without the upper facial angle truly being modified (Figs. 4.4 and 4.5a, b), the mouth is small, with corners that to not exceed the wings of the nose (Fig. 4.6a, b), the palpebral fissures are directed up and behind (Fig. 4.7), the ears are small and low (Fig. 4.8), and the tongue could be protrusive (Fig. 4.9).










Fig. 4.1
Nasal bone not visible at 13 GW (multiplanar view)

Fig. 4.2
Nasal bone not visible at 15 GW (multiplanar view)

Fig. 4.3
Prefrontal edema 22 GW

Fig. 4.4
Flat features

Fig. 4.5
(a) Increased upper facial angle 18 GW; (b) Normal facial angle at 22 GW

Fig. 4.6
Small mouth: (a) 22 GW; (b). 32 GW

Fig. 4.7
Palpebral fissures oriented up and back

Fig. 4.8
Small ear at 26 GW

Fig. 4.9
Protrusive tongue
Nevertheless, in a certain number of cases, the profile could appear normal (Fig. 4.10).


Fig. 4.10
Trisomy 21, normal profile
4.3.2 Trisomy 18
The diagnosis of this chromosomal anomaly is usually based on a collection of morphological anomalies.
In these case, the dysmorphism associates microretrognathia, sloping forehead, and small low ears. Cleft palate is frequent (cf. Clefts)





Fig. 4.11
Microretrognathia at 13GW (multiplanar image)

Fig. 4.12
Microretrognathia at 13GA, rendered image

Fig. 4.13
(a) Sloping forehead and (b) microretrognathia

Fig. 4.14
Small ear
4.3.3 Trisomy 13
Dysmorphism in the case of trisomy 13 is often very spectacular, covering a wide range of possibilities from a simple median incisor (Fig. 4.15) to cyclopia (Figs. 4.16 and 4.17), with a full range of intermediary aspects possible including more or less hypotelorism (Fig. 4.18), single nostrils (Figs. 4.18, 4.19, and 4.20). Clefting is found in 60–80 % of cases, and is both unilateral and bilateral. Median clefts are characteristic (Figs.4.21 and 4.22).
These anomalies are related to cerebral medial line anomalies (alobar, semi-lobar or lobar holoprosencephaly) that are present in 50 % of cases.









Fig. 4.15
Median incisor

Fig. 4.16
(a, b) Cyclopia, proboscis 13GW

Fig. 4.17
Cyclopia, single nostril. 22 GW

Fig. 4.18
Hypotelorism, single nostril 13GW

Fig. 4.19
Single nostril 13GW (multiplanar image)

Fig. 4.20
Single nostril 22 GW:(a): Render 3D, (b): 2D

Fig. 4.21
Median cleft 14 GW

Fig. 4.22
Various fetal facial dysmorphic features related to trisomy 13
Facial dysmorphism related to other chromosomal anomalies are often rougher, as seen in the below examples corresponding to:




Fig. 4.23
Partial trisomy eight profile

Fig. 4.24
Partial trisomy eight (post-natal aspect)

Fig. 4.25
Distal monosomy 6p
4.4 Upper Face Pathologies
4.4.1 Wolf-Hirschhorn Syndrome
Wolf-Hirschhorn syndrome results from a microdeletion, with an incidence of 1/50,000. The deletion occurs on the short arm of chromosome 4 at the 4p.16.3 region. This deletion is sporadic in the majority of cases. Research for this deletion should be signaled to geneticists when they test the amniotic fluid.
The dysmorphism is characteristic:

On the profile, the upper facial angle measurement is barely inferior to normal measurements. However, the glabella appears prominent with a frequently not very echogenic aspect (the prefrontal tissues), which results in a characteristic “Greek Helmet” look (Fig. 4.26).
The corners of the lips appear to be falling.
There can also be hypertelorism, dysplastic ears with periauricular tags, cleft lip or labio-alveolar-palatal clefting.

Fig. 4.26
“Greek Helmet” look
This dysmorphism occurs in a context of early IUGR with, in particular, severe microcephaly, mental retardation could be severe with walking and language acquisition occurring more or less late.
4.4.2 Craniostenosis
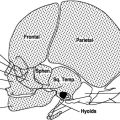
These anomalies, resulting from the premature fusing of one or several sutures, often lead to severe dysmorphism. The overall incidence is estimated at 1/2000 and around 100 different forms have been described. The role of an ultrasonographer consists of labeling as best as possible the type of anomaly and then looking for other markers to determine whether or not the craniostenosis is related to a syndrome. It is useful to remember that the first sign signaling craniostenosis could be an enlargement of a healthy contralateral suture.
Craniostenoses are classified based on the type of suture involved (Figs. 4.27, 4.28, 4.29, and 4.30).


Fig. 4.27
Classification of craniostenoses
Below are a few examples of an approach to these complex pathologies.




Fig. 4.28
Trigonocephaly (premature fusing of the metopic suture)

Fig. 4.29
“Cloverleaf” skull with intra cranial marks (premature fusing of coronal sutures)

Fig. 4.30
Asymmetric craniostenosis (premature fusing of a coronal suture)
4.4.2.1 Examples of Syndrome Craniostenosis
Crouzon syndrome
This syndrome results in the premature closing of the coronal sutures. Facial dysmorphism is characterized by branchycephaly, with a very domed appearance, frontal bumps, moderate hypoplasia of the mid-level face (Figs. 4.31, 4.32, 4.33, and 4.34), proptosis related to shallow orbits (Fig. 4.33), slight hypertelorism (Fig. 4.33), and a straight mandible (Figs. 4.33 and 4.34). Intelligence is generally normal. Complications such as atrophy of the optic nerves and deafness can occur if not caught and treated early. It is an autosomal dominant genetic disorder.





Fig. 4.31
Crouzon syndrome (multiplanar image)

Fig. 4.32
Crouzon syndrome: profile

Fig. 4.33
Crouzon syndrome: “render”

Fig. 4.34
Crouzon syndrome, with very wide metopic suture. (a) Maximum mode; (b) Post-natal
Apert syndrome
This results in the premature closing of the coronal sutures, with broad bone defects that can be seen on the median line between the two fontanels. Dysmorphism is characterized by branchycephaly, a very marked nasal bridge (Fig. 4.35), moderate mid-level face hypoplasia, hypertelorism and a prominent forehead. The hands show syndactyly that leaves the thumb free, producing “mitten” hands (Fig. 4.36), however syndactyly of the feet impacts all the toes.



Fig. 4.35
Apert syndrome: profile

Fig. 4.36
Apert syndrome: dysmorphism and syndactyly producing “mitten” hands
4.4.2.2 DiGeorge Syndrome
This syndrome is linked to a microdeletion at 22.q.1.1 and occurs at a frequency of 1/4000. Transmission is dominant; one parent is carrier of the deletion in 10–20 % of cases, most frequently this deletion is “de novo”.
The main markers are:
Conotruncal heart disorders (75 %) of cases
Hypoplasia or absence of the thymus (in 83 % of cases if there is a heart malformation, and in 31 % of cases if the deletion is isolated)
Palate defects (76 % of cases: submucosal cleft palate and cleft palate, velopharyngeal incompetence, etc.)
Renal anomalies (35 % of cases)
Limb anomalies (hyperlax hands or fingers, polydactyly)
A broad range of psycho-motor development anomalies (slow learning with IQ variations from normal to seriously retarded, psychiatric disorders in 10 % of cases).
The dysmorphism is very moderate, association a “tubular”-looking nose (Figs. 4.37, 4.38, and 4.39), a relatively narrow mouth (Fig. 4.39), and minimal external ear anomalies (Fig. 4.40).





Fig. 4.37
DiGeorge syndrome: multiplanar image, tubular nose (image B)

Fig. 4.38
DiGeorge syndrome: tubular nose, “render”

Fig. 4.39
DiGeorge syndrome: tubular nose, small mouth (Render HD-Live)

Fig. 4.40
DiGeorge syndrome: ear: excessively folded helix
4.4.2.3 Microcephaly
Microcephaly is a clearly defined biometric anomaly: the cranial circumference is smaller than −3 SD or Z-Score : −3. The frequency of 1/6200 to 1/8500 is certainly underestimated. It can be isolated, related to holoproscencephaly, or associated with chromosal anomalies, a number of genetic syndromes, and other morphological anomalies.
Three conditions are required to consider microcephaly:
The dating of the pregnancy and the biometric definition must be precise, and the measuring technique must be irreproachable.
When microcephaly is discovered, on must:
Correlate the cranial circumference with other biometric parameters.
Look for a vascular context.
Test for infections.
Do karyotype testing for a 4p- deletion
Do a family survey
Do a detailed ultrasound, particularly of the cerebral structures.
Dysmorphism primarily takes the form of a sloping forehead (Figs. 4.41, 4.42, and 4.43) and a disproportion between the size of the face and the skull (Figs. 4.42 and 4.43).




Fig. 4.41
Microcephaly: normal-sized face, sloping forehead

Fig. 4.42
Microcephaly: sloping forehead, narrow skull with retrognathia

Fig. 4.43
Microcephaly: “render”
4.5 Mid-Face Pathologies
4.5.1 Achondroplasia
Achondroplasia is the most frequent osteochondrodysplasia, with an estimated frequency between 1/15,000 and 1/26,000. It is related to a mutation of the FGFR3 gene. This bone pathology becomes apparent starting at 26 gestational weeks, the diagnosis most frequently made during the third trimester ultrasound showing very short limbs, in particular rhizomelia contrasting with macrocrania (very marked frontal bossing), and a relatively narrow thorax (Fig. 4.45). In addition to these markers, the ultrasound will show a characteristic appearance of the upper femoral metaphyses, which appear to be slender like “ice cream cones,” leading to an “opening” of the upper femoral angle (Fig. 4.44), short trident hands, and platyspondyly (Fig. 4.45).



Fig. 4.44
Achondroplasia: slender upper femoral metaphysis (2D and 3D)

Fig. 4.45
Achondroplasia: trident hand, narrow thorax, platyspondyly
Dysmorphism combines marked frontal bossing and a very “hollowed” nasal bridge with an abnormally closed upper facial angle (Figs. 4.46, 4.47, 4.48, and 4.49).





Fig. 4.46
Achondroplasia: multiplanar view

Fig. 4.47
Achondroplasia: closed upper facial angle

Fig. 4.48
Achondroplasia: dysmorphism “Render HD Live”

Fig. 4.49
Achondroplasia: dysmorphism “Render”
4.5.2 Maxillo-Facial Dysplasia: Binder Syndrome
This anomaly has an estimated incidence of 1/10,000, but is probably underestimated. A few family recurrences suggest autosomal recessive transmission or low-penetrating dominance. Binder-like dysmorphism can be found in cases of mothers taking phenytoin and anti-vitamin K type anticoagulants, or with lupus.
This sequence is considered to be an allelic variation of punctate chondrodysplasias.
Dysmorphism takes the form of a flattened midface that translates into an abnormally open upper facial angle, a short nose with a flattened root, a slightly protruding mandible, and a little developed upper maxilla (Figs. 4.50, 4.51, 4.52, 4.53, and 4.54). Tests should include karyotype, maternal blood test for lupus, a detailed ultrasound looking for punctate epiphyses (Figs. 4.55 and 4.56), a scan could be done to confirm the ultrasound results. Discovery of a brachytelephalangy would suggest a benign form of chondrodysplasia punctate (Fig. 4.57).









Fig. 4.50
Binder, multiplanar view

Fig. 4.51
Binder, open upper facial angle

Fig. 4.52
Binder, rendered mode

Fig. 4.53
Binder, Render HD-live mode

Fig. 4.54
Punctate calcifications: prenatal ultrasound and corresponding post-natal x-ray

Fig. 4.55
Punctate calcifications – post-natal appearance

Fig. 4.56
Brachytelephalangy: prenatal ultrasound appearance and corresponding post-natal x-ray

Fig. 4.57
Binder, post-natal appearance
4.5.3 Thanatophoric Dysplasia
This is the most frequent of fatal osteochondrodysplasias, with an estimated 1/20,000 to 1/60,000.
Currently, a diagnosis is made most often during the first trimester, although it could be made during the second trimester. It combines very short limbs with femurs shaped like old-fashioned telephone receivers, a narrow thorax that looks like a champagne cork on a sagittal view, platyspondyly with H-shaped vertebrae, and macrocrania. Hydramnios is common (Fig. 4.58).
Dysmorphism is marked by macrocrania, mid-face hypoplasia translated by a marked nasal bridge with a reduced upper facial angle (Figs. 4.59, 4.60, 4.61, and 4.62).






Fig. 4.58
Thanatophoric dysplasia: (a) narrow thorax. (b) short curved femur. (c) platyspondyly. (d) macrocrania

Fig. 4.59
Thanatophoric dysplasia: multiplanar view

Fig. 4.60
Thanatophoric dysplasia: reduced upper facial angle

Fig. 4.61
Rendered image of thanatophoric dysplasia

Fig. 4.62
HD live rendered image of thanatophoric dysplasia
4.5.4 Prader-Willi Syndrome
This rare syndrome affects between 1/10,000 and 1/25,000 births, its origin being a genetic deletion on the paternal chromosome 15 (at 15q11-q13). It is:
Either de novo
or part of a maternal uniparental disomy.
Prader-Willi syndrome is considered with the discovery of hydramnios and slow growth related to diminished active movements, fetal cardiac disorders, and genital anomalies, and a detailed study of the face could then support the hypothesis.
Dysmorphism in this syndrome is characterized by:


A thin, slightly turned up upper lip
Almond-shaped eyes, narrow forehead
A closed upper facial angle

Fig. 4.63
Prader-Willi Syndrome: closed upper facial angle, thin turned up upper lip

Fig. 4.64
Prader-Willi Syndrome: rendered image
4.5.5 Otopalatodigital Syndrome
This is a very rare syndrome (only around 30 cases recorded), two types of which are described: type 1 and type 2. This genetic disease results from mutations in the FLNA gene associated with skeletal dysplasia (curved long bones, limb anomalies, including fan-shaped toes, bent fingers, syndactyly, polydactyly, etc.), auditory deficit, and characteristic facial dysmorphism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree