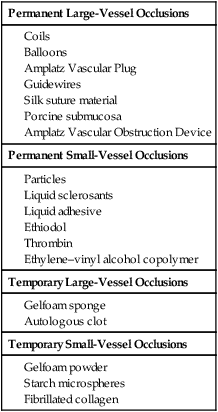
The principle of catheter-directed embolization is to acutely stop flow in a blood vessel or vascular territory by way of mechanical occlusion using a temporary or permanent agent. Along with angioplasty, embolization has been a unique defining procedure in the history of interventional radiology. The elegance of the technique underscores the value of minimally invasive therapy. Since the first reported cases using autologous clot in 1972,1 embolization techniques have evolved with the use of many agents and devices to yield truly impressive results. Traumatic injuries resulted in the first common application of embolization techniques, where until the late 1970s surgery was considered the only viable option for suspected vascular injury. This technique has evolved to include nearly every vascular territory and has been used in such diverse clinical applications as treatment of tumors, vascular malformations, varicosities, aneurysms and pseudoaneurysms, fibroids, and gastrointestinal bleeding. With the increasing number of embolic agents available to the operator, it is often difficult to determine which type of agent would best be used for any given clinical indication. Generally speaking, agents can be separated into one of four types: permanent large-vessel occluders, permanent small-vessel occluders, temporary large-vessel occluders, and temporary small-vessel occluders. The most commonly used embolic agents, separated into these four categories, are presented in Table 10-1. TABLE 10-1 Commonly Used Embolization Materials When deciding which embolic agent is most appropriate for any given application, the authors pose three questions. Is the vessel to be embolized a large vessel (anything that can be seen angiographically to the level of small to medium arterioles) or a small vessel? Is the desired occlusion permanent or temporary? Is cellular or organ death desired? Once these questions are answered, the choice of embolic agent becomes more obvious (Table e10-1). TABLE e10-1 General Embolization Scheme and Clinical Indications AVM, Arteriovenous malformation; UFE, uterine fibroid embolization. Some detachable coils are released when a weld is broken under pressure or electrolytically. For example, GDC Detachable Coils (Cook Medical) and Matrix Detachable Coils (Boston Scientific) have a connection between the coil and delivery wire that “dissolves” when the operator applies a current to the delivery wire. Others, such as the Interlock-35 System and its derivatives (Boston Scientific), feature a mechanical connection between the coil and delivery wire that disengages when the detachment zone at the base of the coil passes beyond the tip of the delivery catheter. Alternatively, some detachable coils use a release mechanism that relies on fine threads at the base of the coil and at the distal end of the delivery wire (or mandril) that are disengaged by rotating the delivery wire. This system was first described with the Jackson detachable coil2 and has evolved to become the Flipper Delivery System (Cook Medical). It has been argued that this system affords better control, since the coil can be retrieved even when the threads have passed beyond the delivery catheter tip. Many of these detachable coils are now available in 0.035-inch diameter. Surface-modified coils of the hydrogel type (HydroCoil Embolic System [MicroVention Inc., Tustin, Calif.]) have recently completed evaluation by a randomized controlled trial.3 These coils employ a thin hydrophilic coating that hydrates and expands when placed intravascularly. Compared to traditional platinum coils, this coating (in principle) leads to better aneurysm occlusion/packing; uncoated platinum coils may leave aneurysms only 20%-30% volumetrically occluded with delivered material, versus approximately 70% with the hydrogel system.4 While the remaining void is filled with coil-induced thrombus, this clot remains unorganized for an extended period of time. It is thought that aneurysm regrowth may be related to thrombolysis and recanalization.3,5 Major aneurysm recurrence (incomplete occlusion on follow-up imaging) is estimated to occur in up to 20% of patients at 6 months after treatment with bare platinum coils.3 Through greater volumetric packing, the hydrogel coils are intended to decrease recanalization and aneurysm recurrence. Whether this will ultimately result in improved clinical outcomes is uncertain. The aforementioned randomized controlled trial noted little difference in terms of bleed or re-bleed, but there were fewer angiographic recurrences in the HydroCoil group.3 Hydrogel-coated coils can be delivered through microcatheters. However, since they expand to a diameter several times their initial measurement, they can only be retracted into the microcatheter for a few minutes following contact with blood, reaching maximum diameter approximately 20 minutes after exposure.4 Complication rates appear to be similar between HydroCoils and traditional coils,3,5 though there has been concern regarding a statistically nonsignificant increased occurrence of hydrocephalus.3 Additionally, some authors initially reported increased rates of thromboembolic complications (primarily clot on coil) before systematically adding aspirin to full heparinization during the embolization procedure.5 HydroCoil steaming/softening before deployment has also been necessary to decrease coil stiffness prior to delivery, though newer less stiff coils are now available.5 In the past, balloons were used in different clinical scenarios where a permanent, proximal, large-vessel occlusion was desired. They especially made their mark as occluders during pulmonary arteriovenous malformation (AVM) embolizations.6 In a small percentage of balloons, deflation occurred post embolization. This phenomenon, coupled with the success and ease of use of coils, led to the removal of balloons from the commercial market in the United States. Described in animal studies as an embolic agent for abdominal aortic aneurysm endoleaks7 and as the membrane for a novel endovascular occluder,8 the material has yet to be used in large published human trials. The SIS material is composed largely of proteoglycans, collagen, and glycosaminoglycans.7 There are no published reports that describe anything other than a simple mechanical occlusion (e.g., no notable inflammatory response), and the occlusion appears to be permanent (D. Pavcnik, personal communication). Per the investigators, when used, it has a consistency similar to Gelfoam. Detailed description of how to prepare the agent is given elsewhere,7 but presumably, prior to arriving on the market for general use, the process of preparation will become less onerous.
Embolization Agents
Types of Agents Used
Permanent Large-Vessel Occlusions
Permanent Small-Vessel Occlusions
Temporary Large-Vessel Occlusions
Temporary Small-Vessel Occlusions
Permanent
Temporary
Large vessel
Coils (e.g., pulmonary AVM)
Gelfoam sponge (e.g., trauma)
Small vessel
Particles (e.g., UFE); no organ death
Liquid agents (e.g., renal ablation); organ death
Gelfoam particles, fibrillated collagen (e.g., chemoembolization)
Permanent Large-Vessel Occlusions
Coils
Balloons
Small Intestine Submucosa
Embolization Agents