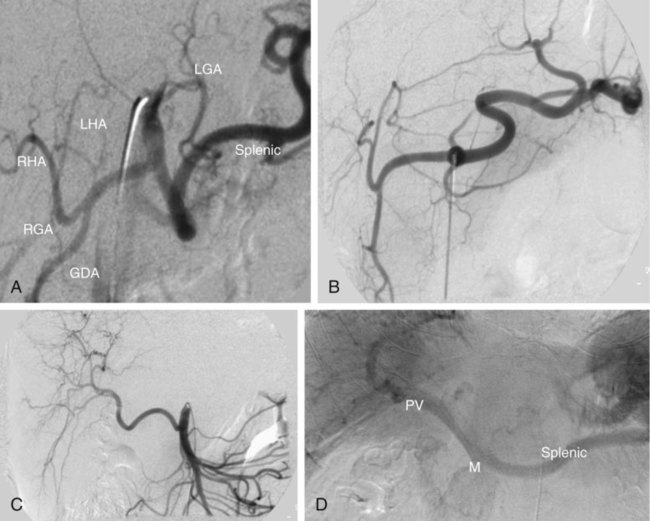
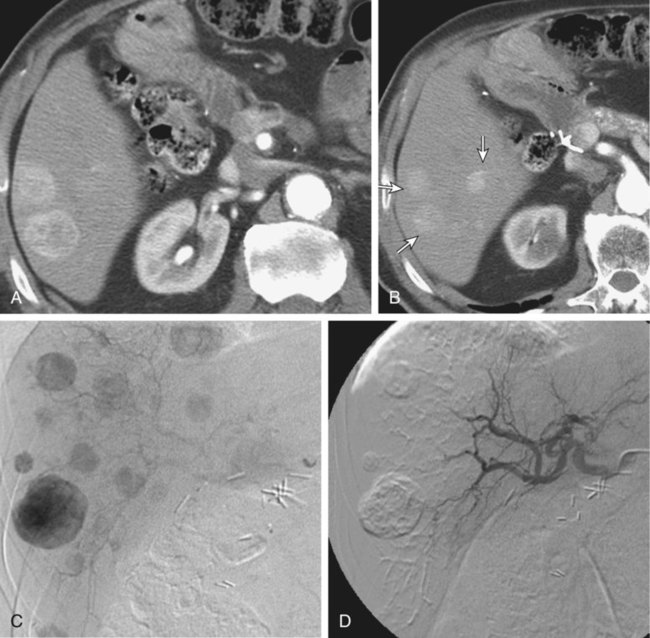
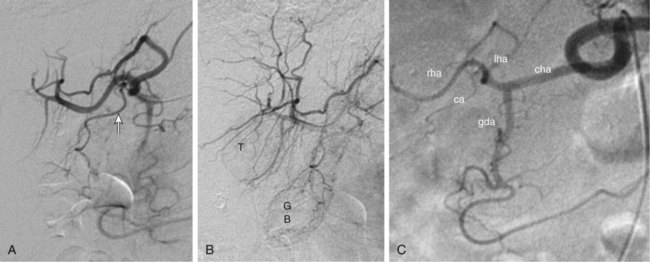
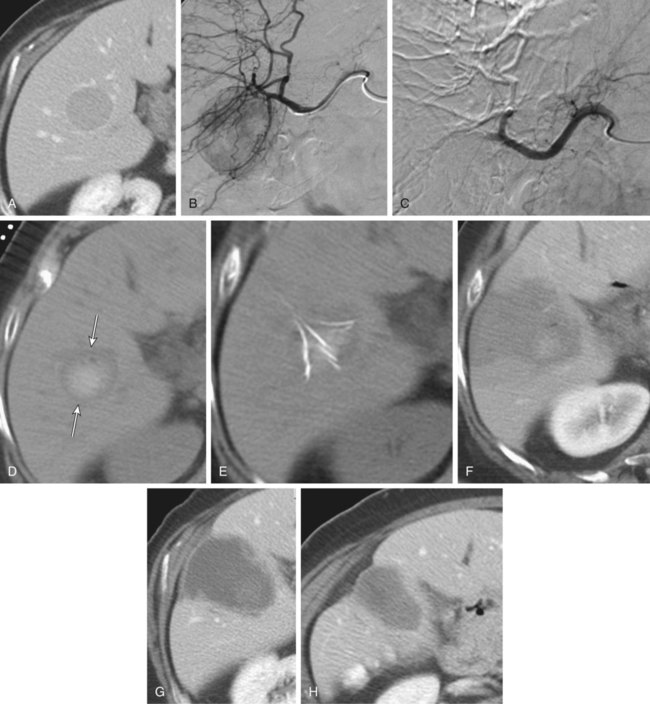
Bland hepatic transcatheter arterial embolization (HAE) and chemoembolization (TACE) are routinely used to treat inoperable hepatocellular carcinoma (HCC)1,2 and other primary and secondary hepatic malignancies.2–42 Embolic treatment of hepatic metastases is undertaken when liver disease is dominant, with no or stable extrahepatic disease. The liver is an ideal organ for arterial embolization in managing tumors supplied by the hepatic artery because it affords selective treatment of the tumor and preserves the blood supply to the organ, at least 70% of which originates from the portal system.1,2 Selective embolization of arterial feeders to the tumor results in ischemia and selective necrosis while sparing the normal liver parenchyma supplied by the portal vein.1–3 HAE used as sole treatment or combined with chemotherapeutic agents (TACE) is targeted to the hepatic arterial branches feeding the tumor. This is achieved using embolic agents (particles) small enough to reach and occlude the capillaries to the tumor. Addition of chemotherapy to embolization (TACE) was based on the theoretic advantage of delivering concentrated drug to a tumor sensitized by the ischemia caused by embolization. Chemotherapeutic dwell time in the tumor is significantly prolonged, and systemic effects are minimized.38 To date, no randomized trial has shown any advantage of TACE over HAE,43 suggesting that the main mechanism of tumor death is the ischemia caused by embolization. Drug-eluting bead (DEB) TACE has been used to treat metastatic colorectal carcinoma, metastatic neuroendocrine tumor, and cholangiocarcinoma. However, there is a lack of standardized comparative data regarding response.44 A small retrospective single-center series by Ruutiainen et al. concluded that there is a trend (not statistically significant) toward improved survival and tumor and symptom control with TACE when compared to hepatic arterial embolization (HAE).45 However, the small numbers, the retrospective nature of the study, a significant patient crossover between the groups, and the relatively larger size of the embolic material used for HAE raise important questions regarding the validity of any meaningful interpretation of that study. Proximal arterial occlusion is not desirable because it will lead to development of intrahepatic collateral vessels that will resupply the tumor.46 Permanent proximal arterial occlusion (surgical ligation or catheter coil embolization) should never be performed in the management of hepatic tumors because it not only provokes intrahepatic collateral formation but also precludes repeat intervention and embolization (Table 66-1). TABLE 66-1 Malignant Liver Tumors Other Than Hepatocellular Carcinoma In general, the selection criteria for patients with HCC apply to patients with other liver malignancies when determining candidates for embolotherapy.1–3 Patients with unresectable malignant tumors supplied by the hepatic arterial tree will benefit from embolization. The purpose of hepatic embolization is either symptomatic relief or improvement of survival. Indications for HAE include rapid progression of liver disease (in the face of stable or absent extrahepatic disease), symptoms related to tumor bulk, and symptoms related to hormonal excess, especially in the case of neuroendocrine tumors (NETs).47 In patients with hepatic metastases from colon, breast, and renal cell carcinoma, melanoma, gastrointestinal tumors or sarcomas, or other primaries, embolization is used for control of tumor load involving the liver to improve outcomes. Control of hepatic metastases may allow prolonged survival when compared with patients who do not undergo embolization.43,46 With the exception of NETs, patients with secondary hepatic disease who have no symptoms related to the hepatic disease but have extrahepatic disease are probably poor candidates for embolization. There are a limited number of contraindications to hepatic embolization for treating liver malignancies. Advanced cirrhosis and liver failure are contraindications to embolization in patients with HCC but are relatively rare in patients with other primary or secondary liver malignancies. Extensive and progressive extrahepatic disease is a contraindication to embolization of hepatic malignancies (with the exception of NETs, where embolization is aimed at controlling hormonal symptoms or pain). Involvement of more than 75% of the liver parenchyma by tumor makes a patient vulnerable to embolization and at high risk for development of liver failure. Although this finding is not an absolute contraindication to treatment, caution should be used and embolization should be performed in stages (one sector at a time).6,16,47 To optimally perform embolization, a dedicated angiography suite is required and must have the capability of digital subtraction angiography (DSA) with the application of road mapping techniques. A fully equipped suite will also have available the appropriate angiographic catheters and microcatheters and corresponding guidewires. A 6F sheath is always needed to make the necessary catheter exchanges with the minimal possible trauma to the common femoral artery. The most commonly used catheters for angiography are 4F and 5F selective Cobras, which nowadays are available in hydrophilic form. Frequently, a 3F microcatheter is used coaxially for superselective embolization. An extensive inventory of guidewires and selective catheters should be available (Table 66-2) for cases in which catheterization cannot be achieved with the Cobra catheter and a 0.035-inch Glidewire. Our contrast agents of choice are iohexol 140 and 300. TABLE 66-2 Technical Aspects: Key Equipment for Hepatic Arteriography and Embolization It is essential that the operator be very familiar with hepatic arterial anatomy and variations (Fig. 66-1, A–C). Before undertaking any type of hepatic embolization, a triphasic contrast-enhanced computed tomography (CT) examination is essential to demonstrate tumor size, location, and arterial supply. It is common to identify replaced or accessory hepatic arterial branches that feed the target tumor and must therefore be embolized. During arteriography and after imaging the celiac axis with selective injections, one should always image the proximal superior mesenteric artery in search for accessory and replaced hepatic arteries (see Fig. 66-1, B and C). It is important to remember that phrenic branches are frequently tumor feeders, particularly in patients who have undergone multiple previous embolization sessions. Embolized tumors frequently recruit the phrenic arteries as an alternative feeding pathway after embolization of the hepatic branches. Before embolization, it is important to assess portal vein status, which can be accomplished with contrast-enhanced CT before the procedure, and arterial portography during arteriography before embolization. Arterial portography is routinely performed with the angiographic catheter in the splenic or superior mesenteric artery and delayed filming, so that the portal vein is opacified and imaged (Fig. 66-1, D). It is imperative that before any embolization procedure, optimal imaging of the liver be performed to depict the anatomy and characteristics of the tumor to be embolized. In our institution, the preferred imaging study before any embolization procedure is a triphasic CT scan. This allows depiction of the arterial anatomy, including the identification of anatomic variations, which is extremely important before any attempt at embolization. It also demonstrates portal vein status. A good triphasic CT scan will also provide information about the vascularity and distribution of the tumor or tumors to be treated (Fig. 66-2, A and B). Angiography and embolization are performed in standard fashion via the femoral artery, with angiography used to depict the hepatic arterial anatomy and variations, as well as the distribution of vascular masses throughout the liver (Fig. 66-2, C). Celiac axis and hepatic arteriography is always performed before embolization. Selective arteriography is necessary to identify tumors with a poor vascular component and, rarely, even hypervascular tumors that were not visualized on the celiac arteriogram. The catheter is advanced over a guidewire in the vessels expected to be supplying the target tumor, based on prior triple-phase CT information. When treating patients with multifocal bilobar disease (see Fig. 66-2, C), we select the lobe with the most disease for treatment at the first sitting. Initially, we perform arteriography with the catheter positioned selectively in the right or left hepatic artery, followed by embolization (Fig. 66-2, D). Several embolic agents are used for treatment, and even more regimens for the administration of TACE. In the following section, we describe the technique of bland embolization we routinely use in our institution. Selection of the embolic agent for treatment is based on the preference of the interventional radiologist. We always start by using the smallest size of the desired particles (see Table 66-2). A vial of the smallest particle is poured into a small glass or stainless steel cup, and the vial is rinsed of residual particles by filling it with contrast material and then pouring the contents into the cup containing the particles. The suspension is made and then drawn into a syringe for administration into the target vessel under continuous fluoroscopic control to confirm antegrade flow toward the tumor until stasis is evident or until 10 mL of the embolic agent has been used. We evaluate flow after the administration of 10 mL of the smallest particles, and if antegrade flow is still evident, we continue embolization with the next smallest particles. Once again, 10 mL of these particles is administered, and if antegrade flow persists, the next size particles are used, again injecting up to 10 mL of the particles, and so on. When stasis (defined as lack of antegrade flow, with evidence of reflux on injection of even small amounts of contrast material) is achieved, we terminate the embolization and perform arteriography to document occlusion of the target vessel and identify any supply to the target tumor from other vessels, which we may then select and embolize. Finally, arteriography will show stasis in the embolized vessel, which will have a “pruned tree” appearance (see Fig. 66-2, D), and preservation of antegrade blood flow to nontarget vessels such as the gastroduodenal and, if visualized, the cystic artery (Fig. 66-3). We recommend and try to embolize solitary tumors (Fig. 66-4, A and B) superselectively with a coaxial technique and 3F microcatheters, again administering up to 10 mL of the smallest particle size available and following the same algorithm as described in the previous paragraph for lobar embolization. We repeat the same procedure until all vessels supplying the tumor are occluded according to our standard protocol, beginning with the smallest particles and continuing until stasis. As with any type of embolization, we perform a final arteriogram when stasis has been achieved in all target branches (Fig. 66-4, C). At this point we consider evaluation of vessels that have not been embolized, and if appropriate, we also perform angiography of nonhepatic vessels supplying the tumor (e.g., phrenic or internal mammary arteries). Provided the patient is doing well, excessive contrast agent has not been administered, and embolization of additional vessels is considered safe, embolization continues. When stasis has been achieved in all vessels supplying the target tumor or the hemiliver, embolization is concluded, and when necessary, a future treatment plan for repeat embolization of residual disease is devised. Patients with up to three tumors less than 5 cm in size (see Fig. 66-4, A) usually undergo ablation after embolization. Radiofrequency ablation (RFA) is performed unless contraindicated due to proximity (<1 cm) to structures that may be in danger from thermal injury. This clinical protocol aims to achieve “double kill” of the tumor. We believe combining the two methods increases the possibility of achieving 100% tumor cell death. The combination of arterial embolization and thermal ablation makes sense in that embolization eliminates the arterial blood supply, thereby removing at least the arterial “heat sink” and allowing the maximum effect of RFA. In addition, bland embolization frequently results in dense opacification (Fig. 66-4, D) of the treated tumor by the combination of particles mixed with contrast material that remains within the treated tumor, very similar to what is seen after TACE with Lipiodol. This facilitates targeting of the lesion by CT the day after embolization, and coverage of the entire lesion with the appropriate-size RFA probe (Fig. 66-4, E), as well as performance of additional overlapping treatment to provide a clear margin of necrosis around the treated lesion (Fig. 66-4, F–H).
Embolotherapy for the Management of Liver Malignancies Other Than Hepatocellular Carcinoma
Primary
Metastatic
Cholangiocarcinoma
Neuroendocrine tumor
Angiosarcoma
Sarcoma, gastrointestinal stromal tumors
Melanoma and ocular melanoma
Colorectal cancer
Breast cancer
Renal cell cancer
Indications
Contraindications
Equipment
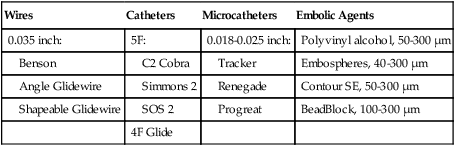
Wires
Catheters
Microcatheters
Embolic Agents
0.035 inch:
5F:
0.018-0.025 inch:
Polyvinyl alcohol, 50-300 µm
Benson
C2 Cobra
Tracker
Embospheres, 40-300 µm
Angle Glidewire
Simmons 2
Renegade
Contour SE, 50-300 µm
Shapeable Glidewire
SOS 2
Progreat
BeadBlock, 100-300 µm
4F Glide
Technique
Anatomy and Approach
Preprocedural Preparation
Technical Aspects
Tumors Smaller Than 5 cm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree