Endocrine Gland Scintigraphy
Marc G. Cote
Thyroid
Imaging Methods. Diagnosis and treatment of thyroid disease requires the evaluation of thyroid function, anatomy including palpatory findings with or without thyroid US, and tissue characterization of thyroid lesions (1,2,3). Radionuclide scintigraphy and measurement of radioiodine uptake form the basis of functional assessment of the thyroid. Functional imaging combined with serological serum levels of thyroid hormones allows for the determination and classification of thyroid disease.
Radionuclide scintigraphy is used to assess the physiologic function of the gland and to determine the presence or the functional status of thyroid nodules post–fine needle aspiration (FNA). Thyroid imaging is most commonly indicated to evaluate hyperthyroidism. Solitary thyroid nodules are best evaluated initially with FNA. Radionuclide scintigraphy using I-123 is useful in differentiating substernal thyroid from thymus glands. Recently, the complimentary use of single-photon emission computed tomography with computed tomography (SPECT/CT) has allowed for a better localization of abnormal areas of focal uptake in iodine-avid thyroid cancer (4). Single-photon positron emission tomography with computed tomography (PET/CT) has emerged as a useful adjunct to iodine scintigraphy when evaluating for the reoccurrence of poorly differentiated noniodine-avid thyroid cancer (5).
Normal thyroid parenchyma appears relatively homogeneous with technetium-99m-pertechnetate (Tc-99m-O4) or Iodine-123 (I-123) scintigraphy. Iodine is trapped via active transport and organified onto the tyrosine contained in the intrathyroidal thyroglobulin within the thyroid follicles. Tc-99m-O4 is only trapped and will subsequently wash out of the gland since it is not organified. I-123 is the radiopharmaceutical of choice for thyroid imaging especially when imaging nodules (Table 57.1). Tc-99m-O4 is best reserved for imaging hyperthyroid patients in conjunction with an Iodine-131 (I-131)-radioactive iodine uptake (RAIU—the percentage of the administered dose present in the thyroid gland at a specific time after oral administration, usually obtained at 4 and 24 hours).
The functional status of a thyroid nodule may be categorized as hyperfunctioning (“hot”), hypofunctioning (“cold”), or indeterminate (sometimes called “warm”) relative to the normal parenchymal uptake of radioiodine. The term “warm” is misleading to clinicians and should not be used. Hot nodules usually represent hyperfunctioning adenomatous tissue and are rarely malignant. Although solitary cold nodules are hypofunctioning adenomatous tissue in approximately 40% of cases, they may harbor malignancy in up to 15% of cases (6). Indeterminate nodules have the same significance as that of cold nodules. The term “warm” should be avoided since it is easily misunderstood by the referring health-care provider to have the same clinical significance as that of a “hot” nodule. Indeterminate nodules are due to normal activity overlying or surrounding a hypofunctioning cold nodule.
Tc-99m-O4 is inexpensive but has the disadvantage of a lower target-to-background ratio. Tc-99m-O4 has an additional disadvantage in not excluding discordant nodules requiring an additional I-123 study to exclude a discordant nodule. A discordant nodule demonstrates increased Tc-99m-O4 uptake but decreased I-123 uptake, signifying that the nodule could potentially harbor malignancy. Physiologically, discordant nodules still have the ability to trap Tc-99m-O4 but have lost their ability to organify and retain iodine. Since pertechnetate imaging is performed 4 to 6 hours after administration, initial trapping of the radiopharmaceutical may reveal uptake that is isointense or is increased relative to normal parenchyma. I-123 imaging is performed 18 to 24 hours after administration. Any iodine which may have been trapped has time to wash out of the gland prior to imaging, thus revealing the true nature of the nodule.
Although US is superior to palpation (7) in detecting nonpalpable thyroid abnormalities, it is possible to detect nonpalpable abnormalities using a gamma camera with a pinhole collimator. Anatomical abnormalities smaller than 1 cm cannot be reliably resolved because of the inherent limitations of the Anger camera. Thyroid US has largely replaced this role of scintigraphy since studies that compared palpation of the gland with US demonstrated the relative insensitivity of palpation to nodule size (7,8). Incidentalomas found on CT scans performed for other indications detect a number of thyroid nodules.
Historically, RAIU served as a measure of thyroid function for many years prior to the development of laboratory assays. The development of accurate serological methods of measuring serum levels of thyroid hormones and ultra sensitive third and fourth generation thyroid stimulating hormone (TSH) assays
(9) provides a superior method of evaluating thyroid function. Serum TSH is the single best test for screening thyroid function. Only in cases of suspected pituitary or hypothalamic disease is the TSH alone insufficient for screening the thyroid functional status. Measurement of the RAIU is usually indicated for one of the three reasons: (1) differentiating Graves disease (uptake high, usually >35% at 24 hours) from subacute or factitious hyperthyroidism (uptake usually <2%), (2) assisting in the calculation of radioactive iodine dose for the treatment of Graves disease, and (3) assessing suspected toxic multinodular goiters.
(9) provides a superior method of evaluating thyroid function. Serum TSH is the single best test for screening thyroid function. Only in cases of suspected pituitary or hypothalamic disease is the TSH alone insufficient for screening the thyroid functional status. Measurement of the RAIU is usually indicated for one of the three reasons: (1) differentiating Graves disease (uptake high, usually >35% at 24 hours) from subacute or factitious hyperthyroidism (uptake usually <2%), (2) assisting in the calculation of radioactive iodine dose for the treatment of Graves disease, and (3) assessing suspected toxic multinodular goiters.
Table 57.1 Radiopharmaceuticals Used for Thyroid Imaging | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
If the 24-hour RAIU is to be performed using I-131, 5 to 10 μCi is administered orally but no imaging is possible at this RAIU dose. Alternately, I-123 administered orally may be used to perform both the imaging and the uptake studies using a dose of 200 to 400 μCi. For the RAIU uptake, a nonimaging uptake probe is used to obtain counts in a neck-phantom standard. At 24 hours, counts are obtained from the patient’s neck for thyroid counts and counts are obtained from the thigh to determine the background activity. Many laboratories also count the patient at 4 to 6 hours so that markedly hyperthyroid rapid turnover patients are not missed. Rapid turnover patients show a markedly elevated 4- to 6-hour RAIU (25% to 50%) but a lower if not normal 24-hour RAIU. Rapid turnover is seen in the setting of marked Graves disease when the small dose of radioactive iodine is rapidly organified and released into the blood stream as thyroid hormone and subtracted with the thigh background counts.
where cpm = counts per minute. Normal = 10% to 30% at 24 hours (highly dependent on iodine intake).
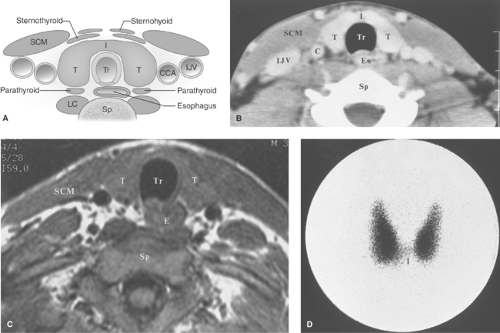
Anatomy, Physiology, and Embryology. The thyroid is located in the lower part of the neck. It consists of two lobes of approximately equal size (5 × 2 cm) positioned on either side of the trachea and connected across the midline by the thin thyroid isthmus inferiorly (Fig. 57.1). A mild degree of asymmetry in the size of the lobes is common. The lobes of the thyroid lie between the carotid artery and the jugular vein laterally and the trachea medially. They rest on the longus colli muscles posteriorly and are covered by the sternohyoid, sternothyroid, and the prominent sternocleidomastoid muscles anteriorly. A pyramidal lobe (normal variant) extends upward from the isthmus or most commonly from the left lobe in as many as 40% of individuals and represents a lower thyroglossal duct remnant. Histologically, the thyroid gland is composed of the thyroid-hormone secreting follicular cells arranged in acini, with central collections of colloid. Follicular cells originate embryologically from the endoderm at the base of the tongue (foramen ovale) that descends to their usual position in the neck. Failure of the thyroid to descend may result in a lingual thyroid. Lingual thyroid pediatric patients are at high risk of developing hypothyroidism with an estimated risk of approximately 30%. Thyroglossal duct persistence beyond the second gestational month of the thyroid’s descent tract may occur and result in a persistent thyroglossal duct. Parafollicular cells (“C cells”), which produce calcitonin, comprise a small proportion of the cell population and are predominately located in the superior two-third of the gland. Parafollicular “C” cells, which reside in the connective tissue adjacent to the follicular cells, originate embryologically from the ectoderm, specifically the fourth pharyngeal pouch and the descendants of the amine precursor uptake and decarboxylation (APUD) system. Parafollicular “C” cells, if they become cancerous, are the anatomical origin of medullary thyroid cancer.
The role of the thyroid gland is the production, storage, and release of thyroid hormones. TSH, produced by the anterior portion of the pituitary gland, regulates the thyroid’s production and release of thyroid hormones. TSH secretion is regulated by hypothalamic thyrotropin-releasing hormone (TRH) and suppressed by circulating thyroxine (T4) and triiodothyronine (T3). Dietary iodine is absorbed in the stomach
and the upper small bowel where it is rapidly reduced to iodide. An active transport process in the follicular cells traps iodine from the blood stream where it is incorporated (organified) onto the tyrosine molecule contained in the intrathyroidal thyroglobulin in the production of T4 and T3. Depending upon the dietary food stuffs and their content, approximately 25% of ingested iodine is trapped by the thyroid while the remaining 75% is excreted in the urine. Recommended daily adult allowance for iodine is 100 to 150 mg. Most developed countries exceed the recommendation. For example, the United States daily intake of iodine may contain as much as 500 mg from commercial breads which are fortified with iodine, seafood, and dairy products. Iodine deficiency is still endemic in certain parts of the world, particularly in the Andes, the Himalayas, and the inland areas of Europe and Africa. Various vegetables such as cabbage and turnips, which contain thiocyanates, can compete with iodine uptake since iodine competes with the monovalent anion of pertechnetate, perchlorate, and thiocyanates.
and the upper small bowel where it is rapidly reduced to iodide. An active transport process in the follicular cells traps iodine from the blood stream where it is incorporated (organified) onto the tyrosine molecule contained in the intrathyroidal thyroglobulin in the production of T4 and T3. Depending upon the dietary food stuffs and their content, approximately 25% of ingested iodine is trapped by the thyroid while the remaining 75% is excreted in the urine. Recommended daily adult allowance for iodine is 100 to 150 mg. Most developed countries exceed the recommendation. For example, the United States daily intake of iodine may contain as much as 500 mg from commercial breads which are fortified with iodine, seafood, and dairy products. Iodine deficiency is still endemic in certain parts of the world, particularly in the Andes, the Himalayas, and the inland areas of Europe and Africa. Various vegetables such as cabbage and turnips, which contain thiocyanates, can compete with iodine uptake since iodine competes with the monovalent anion of pertechnetate, perchlorate, and thiocyanates.
Hypothyroidism. In iodine deficient endemic areas, hypothyroidism is usually caused by dietary iodine deficiency with a pathognomonic concomitant goiter (enlarged gland). Chronic thyroiditis (Hashimoto disease) is the most common noniatrogenic cause of hypothyroidism in iodine-replete areas and a goiter is usually clinically evident. Prior treatment of hyperthyroidism with radioactive I-131 is another common cause (no goiter). Neonatal hypothyroidism is due to thyroid dysgenesis (agenesis, hypoplasia, or ectopia). Pediatric lingual thyroid has a 30% chance of developing hypothyroidism with potential consequences to brain development if hypothyroidism is not diagnosed and treated. Hypothyroidism’s usual clinical features include weight gain, cold intolerance, sluggishness, fatigue, and dry skin. Laboratory findings include elevated serum TSH and low serum T4.
Hyperthyroidism. Graves disease (diffuse toxic goiter) is the most common cause of hyperthyroidism. Other causes include subacute or painless thyroiditis, toxic nodular goiter, early phase of postpartum thyroiditis (thyrotoxicosis precedes the subsequent development of transient hypothyroidism), and factitious hyperthyroidism due to ingestion of thyroid-hormone tablets. Hyperthyroidism’s clinical features include weight loss, increased appetite, tremor, heat intolerance, palpitations, muscle weakness, goiter, exophthalmus, and mood changes or irritability. Laboratory findings include a markedly decreased (suppressed) serum TSH and an elevated serum T4.
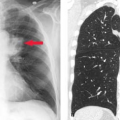
Goiter refers to the clinical finding of generalized thyroid enlargement. Goiter may be associated with increased, decreased, or normal thyroid-hormonal function. Thyroid enlargement may be suspected by physical examination and its accurate extent determined by various imaging techniques, most commonly thyroid US. Goiters extending into the thorax may be best imaged with the use of I-123. Tc-99m-O4 is not useful with substernal goiter due to the large amount of blood-pool activity within the chest.
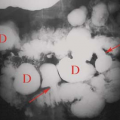
Multinodular goiter is a commonly used clinical term for adenomatous hyperplasia. Imaging studies reveal a diffusely abnormal enlarged nodular gland with a heterogeneous uptake of the radiopharmaceutical or a pattern of multiple discrete
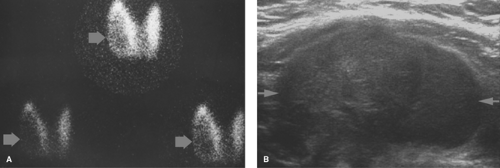
hot nodules on a background of normal or “cool” parenchyma. Photopenic regions should be palpated and dominant palpable nodules should be marked to assure that they do not represent a dominant cold nodule. A 4.1% rate of malignancy occurs in patients with a dominant palpable cold nodule in the setting of multinodular goiter (6). The hot nodules represent autonomously functioning thyroid adenomas, which are usually benign (Fig. 57.2) (7,10).
hot nodules on a background of normal or “cool” parenchyma. Photopenic regions should be palpated and dominant palpable nodules should be marked to assure that they do not represent a dominant cold nodule. A 4.1% rate of malignancy occurs in patients with a dominant palpable cold nodule in the setting of multinodular goiter (6). The hot nodules represent autonomously functioning thyroid adenomas, which are usually benign (Fig. 57.2) (7,10).
Nontoxic goiter may be related to iodine deficiency, excessive consumption of goitrogens in the diet (cooking deactivates goitrogens), medications, or a thyroid enzyme deficiency. The gland is usually soft and symmetric but may appear multinodular with age.
Thyroiditis. All types of thyroiditis are characterized by a rapid asymmetric glandular enlargement, with or without nodularity. Inflammatory changes may fixate the gland to adjacent structures and simulate malignancy. Infection of the thyroid gland may be acute and suppurative because of gram-positive bacteria or subacute because of viral infection, which may involve only a portion of the gland. Immunocompromised patients such as diabetics with multinodular goiters have a greater risk of developing suppurative infections. Suppurative infection is associated with hemorrhage, necrosis, and abscess formation and is a medical emergency since it may transcend into the mediastinum. Subacute viral infection usually causes focal edematous enlargement of the gland. Subacute viral infection may have a protean presentation that mimics some of the clinical features of Graves disease because of the release of all preformed thyroid hormone as a response to the inflammation. The RAIU allows for the differentiation of this syndrome from Graves’ disease. Unlike Graves disease with its high RAIU and intense thyroid-scan appearance, subacute viral patients have a very low RAIU such that scintigraphy of the thyroid gland is rarely indicated. The majority of patients with subacute thyroiditis will resolve and return to a euthyroid state after a transient period of hypothyroidism and elevation of RAIU as the gland returns to normal.
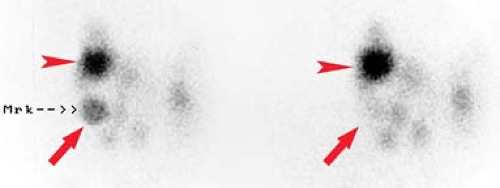
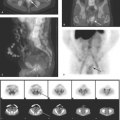
Graves disease is the most common cause of hyperthyroidism. It is an autoimmune disorder in which thyroid-stimulating antibodies cause hyperplasia and hyperfunction of the thyroid gland. The gland is usually enlarged two- to threefold, homogeneous on thyroid scan, and without palpable nodules with elevated RAIU (Fig. 57.3). The treatment of choice for nonpregnant, non–breast feeding adults with Graves’ disease is oral I-131 in conjunction with beta blockers such as propranolol to control symptoms during therapy. Treatment options include subtotal thyroidectomy or antithyroid drugs such as propylthiouracil, methimazole, and carbimazole.
I-131 in the form of sodium iodide has been in the use for many years. It is given by mouth either as a capsule or as a liquid. After uptake by the gland, the high-energy beta particles (mean energy of 0.19 MeV) deliver an average of 1 rad/mCi (1000 rads/mCi) to the thyroid cells. There is very little radiation dose to structures outside the thyroid gland since the average range of the beta particles is 0.8 to 1.0 mm in soft tissue. Most patients will become euthyroid or hypothyroid after a single dose; 10% to 20% of patients require a second or third dose. Patients generally become euthyroid by 10 to 12 weeks after therapy and frequently become hypothyroid by 6 to 12 months. Estimation of the dose of I-131 is empiric. A commonly used formula is:
resulting in a typically administered oral dose of approximately 5 to 20 mCi of I-131. The higher the dose, the quicker the response and the sooner the patient becomes hypothyroid. The smaller the dose, the longer it takes to become euthyroid and the later the development of hypothyroidism. However, it appears that hypothyroidism cannot be avoided, merely delayed by using small doses of I-131 Therefore, it has become a common policy in many centers to give larger doses of I-131 in the range of 15 to 25 mCi with the understanding that hypothyroidism is inevitable and is easily treated with daily replacement levothyroxine. It is important to document with laboratory testing that females of childbearing age are not pregnant prior to treatment with radioactive iodine since iodine crosses the placental barrier and will damage the fetal thyroid. The physician should ascertain that the woman is not breast feeding, since human milk concentrates iodine and the I-131 in her milk would result in exposure to her infant. Many recommend waiting 4 to 6 weeks or more after cessation of breast feeding to allow the breast tissue to involute and decrease breast tissue exposure to I-131.
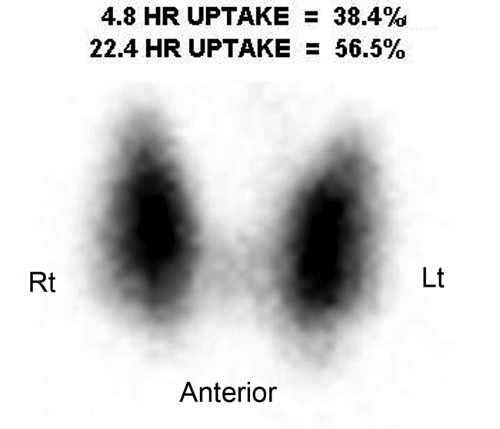 Figure 57.3. Graves Disease. This I-123 scan demonstrates diffuse intense thyroid uptake without cold nodules. Elevated radioiodine uptake is present at 4 hours, 38.4%, and at 24 hours, 56.5%. |
Complications are uncommon. Transient worsening of thyrotoxicosis is, however, fairly common. It occurs a few days to 2 to 3 weeks after treatment and is due to the release of preformed thyroid hormone from disrupted follicles. Occasionally, patients develop symptoms of subacute thyroiditis, with pain and tenderness in the thyroid, often radiating to the ears or the jaw. Temporary hypoparathyroidism and recurrent laryngeal nerve damage have been reported after radioactive iodine treatment but both are exceedingly rare. Though serious
and life threatening, thyroid storm is a very rare complication, more often seen after surgery in inadequately prepared patients. The patient’s risk of genetic damage is no greater than their baseline pretreatment risk provided they wait 6 months prior to conception. Carcinogenesis is not statistically increased. All forms of thyroiditis may be mistaken for tumor because of rapid asymmetric enlargement and nodularity (11).
and life threatening, thyroid storm is a very rare complication, more often seen after surgery in inadequately prepared patients. The patient’s risk of genetic damage is no greater than their baseline pretreatment risk provided they wait 6 months prior to conception. Carcinogenesis is not statistically increased. All forms of thyroiditis may be mistaken for tumor because of rapid asymmetric enlargement and nodularity (11).
Acute (suppurative) thyroiditis is secondary to bacterial infections caused by Strep., Staph., or Pneumococcus. The condition presents with fever, severe sore throat, and asymmetric swelling, and may result in sepsis from hematogenous spread or extend into the mediastinum via fascial planes. Other clinical complications include airway compromise to vascular thrombosis of neighboring vessels. Surgical drainage, antibiotics, and medical management with attention to the airway may be required in these acutely ill patients.
Subacute (viral) thyroiditis has many eponyms but is commonly known as de Quervain or granulomatous thyroiditis. Subacute thyroiditis presents with thyroid pain and hyperthyroidism following an upper respiratory infection as the gland is disrupted and releases its thyroid hormone into the blood stream. Iodine uptake is usually decreased or is absent in the acute stages. The disease runs a subacute course of a few weeks to a few months before healing and returning back to a euthyroid state.
Postpartum thyroiditis typically presents in months 2 to 6 postpartum as a result of nonpainful inflammatory changes. Woman at risk of developing postpartum thyroiditis include those with autoimmune disorders, positive antithyroid antibodies, or previous history of postpartum thyroiditis. Clinically, the early phase manifests as hyperthyroid symptoms with subsequent development of hypothyroid symptoms in the later stage. The majority of patients will return to a euthyroid state within a year with a minority requiring lifelong thyroid-hormone replacement treatment.
Hashimoto thyroiditis is the most common cause of goiter and primary hypothyroidism in adults in developed countries. It is an autoimmune disorder with circulating antithyroid antibody. Histology demonstrates diffuse lymphocytic infiltration of the gland. The thyroid is diffusely enlarged with a rubbery palpable texture. Its early phase is a hyperthyroid-like picture that subsequently evolves into its final hypothyroid sine qua non.
Riedel thyroiditis is a rare inflammatory fibrosing process that involves the thyroid and commonly extends into the neck. Radionuclide uptake is absent (cold) in the involved areas.
Secondary hyperthyroidism may develop in patients with hydatidiform moles or choriocarcinoma. A subunit of the human chorionic gonadotropin (hCG) produced by these conditions demonstrates considerable similarity to TSH thereby directly stimulating the thyroid. Clinical history and serum determination of hCG should be performed if this is a consideration.
Thyroid Nodules
Thyroid nodules are extremely common, while thyroid cancer is relatively rare (12,13). Nodules can be palpated in 4% to 7% of American adults who are asymptomatic for thyroid disease. Autopsy studies demonstrate thyroid nodules in 50% of patients with clinically normal thyroid glands (14). US studies can detect thyroid nodules in 36% to 41% of middle-aged adults (15) with some studies reporting even higher rates (16) of 67%. Thyroid cancer, on the other hand, affects only 0.1% of the population. The incidence of thyroid cancer has increased to ∼37,200 new cases each year (17). Thyroid cancer represents less than 1% of all cancer and is responsible for less than 0.5% of all cancer death. The challenge of clinical evaluation and imaging studies is to establish the likelihood of malignancy and to select for surgery only those patients at a high risk for thyroid malignancy. Recent consensus panels have developed algorithms for the workup and management of thyroid nodules (3).
US is highly sensitive for the detection of thyroid nodules but the specificity for determining malignancy is low. Recent consensus panels have discouraged the routine use of US for screening. Neither MR nor CT improves specificity. This is not surprising since the histological differentiation of a benign follicular adenoma from a well-differentiated follicular carcinoma is based solely on the identification of vascular invasion.
On the basis of radioiodine or technetium pertechnetate uptake during imaging, nodules may be classified as hypofunctioning (cold) (Fig. 57.4), relative to the rest of the gland, hyperfunctioning (hot) (Fig. 57.5), or indeterminate. In a patient with a nodular goiter, the main concern is whether or not thyroid carcinoma is present. Single cold nodules have a 10% to 15% incidence of malignancy, while malignancy is exceedingly rare in hot nodules. A multinodular gland with one or more cold nodules may harbor cancer in up to 5% of patients. If Tc-99m-O4 is used for imaging and a hot nodule is discovered, imaging must be repeated with I-123 as thyroid carcinoma may occasionally trap Tc-99m-O4, resulting in a hot nodule. This is called a discordant thyroid nodule and this nodule would be cold with I-123 imaging.
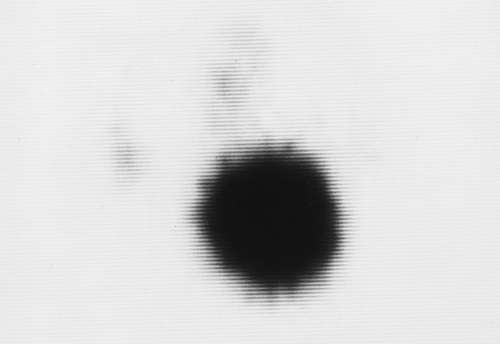 Figure 57.5. Hot Nodule. A hyperfunctioning adenoma demonstrates intense radionuclide activity with the suppression of the function of the remainder of the gland. |
The differential diagnosis of thyroid nodules is as follows:
Follicular adenoma is the most common benign neoplasm of the thyroid and represents about 20% of thyroid nodules. There are many subtypes based upon the histological criteria, including Hürthle cell adenoma, colloid adenoma, and others. Most are solitary, round or oval, and well encapsulated. Regressive changes are extremely common and greatly affect a nodule’s imaging appearance. These include focal necrosis, hemorrhage, edema, infarction, fibrosis, and calcification.
Adenomatous hyperplasia is responsible for up to 50% of thyroid nodules. Adenomatous nodules, also called colloid nodules, are not true neoplasms but are the result of cycles of hyperplasia and involution of a thyroid lobule. They are frequently multiple but one nodule may be dominant. Regressive changes are common including necrosis, hemorrhage, cystic degeneration, and calcification.
Thyroid cysts are extremely rare. Most cystic nodules found in the thyroid are actually cystic degeneration of an adenomatous nodule or a follicular adenoma. The incidence of malignancy within a thyroid cyst (14) is reported to be in the range of 0.5% to 3.0%. Therefore, fluid should be submitted for cytology and the area aspirated should have adequate sampling.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree