EUS in the Esophagus
Esophageal cancer
The TNM classification (Table 11-1) is currently used for the staging of esophageal cancer1,2 and accurate staging is critical for directing patients to appropriate treatment protocols. For instance, 5-year survival is >95% for stage 0 disease, 50–80% for stage I disease, and 10–40% for stage II disease.1–3 EUS has been demonstrated to be most reliable for locoregional staging, staging T3 and T4 lesions with accuracies of 89–94% and 88–100%, respectively.4,5 T1 and T2 lesions can be differentiated from T3 and T4 lesions with an accuracy of 87%.6 EUS is the most accurate modality for staging of regional lymph nodes compared to CT scan and PET.7 The overall accuracy of N staging is 75–80% compared to CT (51–74%).5 FNA improves the diagnostic performance of EUS by at least 13%.8 Unfortunately, EUS’s role for metastases (M) staging has been disappointing. PET scan and CT remain the most accurate methods for M staging. Small liver metastasis may be better detected by EUS.9
Mediastinal masses, nodes, and cysts
EUS is an excellent method of evaluating the posterior mediastinum. The role of EUS in the staging of lung cancer will not be discussed here but it has been used to sample not only primary lung masses but, most importantly, mediastinal lymph nodes in order to determine if there is meta-static involvement.10,11 Lymph node sampling by EUS-FNA can also play a critical role in diagnosis of benign disease such as tuberculosis, lymphoma, sarcoidosis, and histoplasmosis.12,13 Overall, the diagnostic accuracy of EUS-FNA for posterior mediastinal lymph nodes is approximately 93%.14 In addition to primary metastatic lung cancer, posterior mediastinal masses can represent other metastatic disease or primary posterior mediastinal neoplasms, 75% of which are neurogenic tumors.15 Foregut cysts, either bronchogenic, neuroenteric, or esophageal duplication cysts, make up 15% of posterior mediastinal masses and can be differentiated by EUS based on appearance, location, and fluid contents. Sampling by FNA is not highly recommended for simple cysts given an increased risk of infection.16–18 If FNA is performed, then antibiotics are often given prophylactically.19 Ten to 15% of benign mediastinal masses are congenital foregut cysts. Oftentimes mistaken for mediastinal masses, mediastinal cysts can be differentiated from mass lesions by EUS.
Other common esophageal lesions included leiomyomas and granular cell tumors. Esophageal leiomyoma is a benign tumor. It typically originates from the muscularis mucosae, which can be determined by EUS. Granular cell tumors are small neoplasms typically found incidentally on upper endoscopy. The malignancy rate was initially thought to be small;20,21 however, recent reports have suggested their propensity to be infiltrative.22 Endoscopic ultrasound can provide information on the layer of origin and tumor extension. These lesions are usually confined to the mucosal and submucosal layers.
 EUS in the Stomach
EUS in the Stomach
Gastric adenocarcinoma
Gastric adenocarcinoma accounts for more than 90% of all gastric cancers.23 In the United States there has been an increase in the incidence of gastric adenocarcinoma. As with other upper GI cancers, prognosis correlates with resectability. EUS is currently the most accurate method of staging localized gastric cancer,24 therefore permitting the selection of lesions most amenable to endoscopic resection by either endoscopic musocal resection (EMR) or endoscopic submucosal dissection (ESD). High-frequency transducers help characterize the degree of mucosal and submucosal involvement, allowing the differentiation of T1 and T2 lesions in which the tumor infiltrates into the muscularis propria. T2 lesions are no longer considered safe for endoscopic resection and require surgical management.25 N staging of gastric cancer requires careful examination of nodes in the areas of greater and lesser curvatures, the celiac axis, the gastrohepatic ligament, and the splenic hilum. FNA of any suspicious lymph nodes is crucial in confirming malignant involvement. However, EUS remains less accurate for N staging than T staging.24 Cross-sectional imaging remains the favored method to evaluate meta-static disease in gastric adenocarcinoma. If performed, EUS with FNA can help detect distal metastasis in the posterior mediastinum, left adrenal gland, and pancreas, and allow for sampling of ascites.26,27
Gastric lymphoma
Gastric lymphoma comprises <10% of GI malignancies, but is the most common site of gastrointestinal lymphoma.28 Most gastric lymphomas are mucosal-associated lymphoid tumors (MALT) and are usually associated with chronic infection with Helicobacter pylori or with an autoimmune process.28,29 EUS with FNA has a reported accuracy of 89% in the diagnosis of gastric non-Hodgkin lymphoma.30 EUS-FNA, with flow cytometery, can be used to distinguish MALT from other gastric B-cell lymphomas.29
Subepithelial lesions
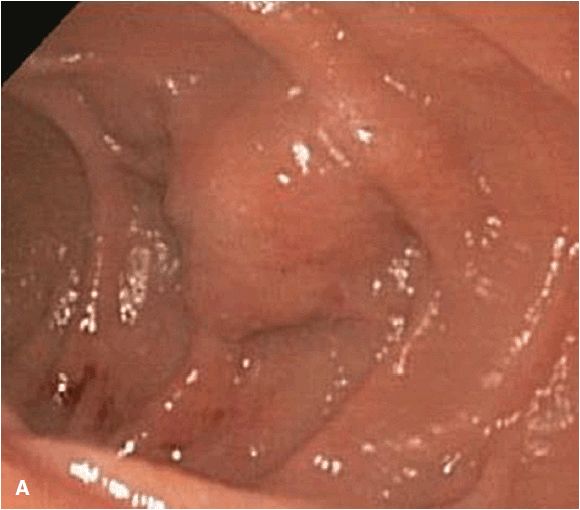
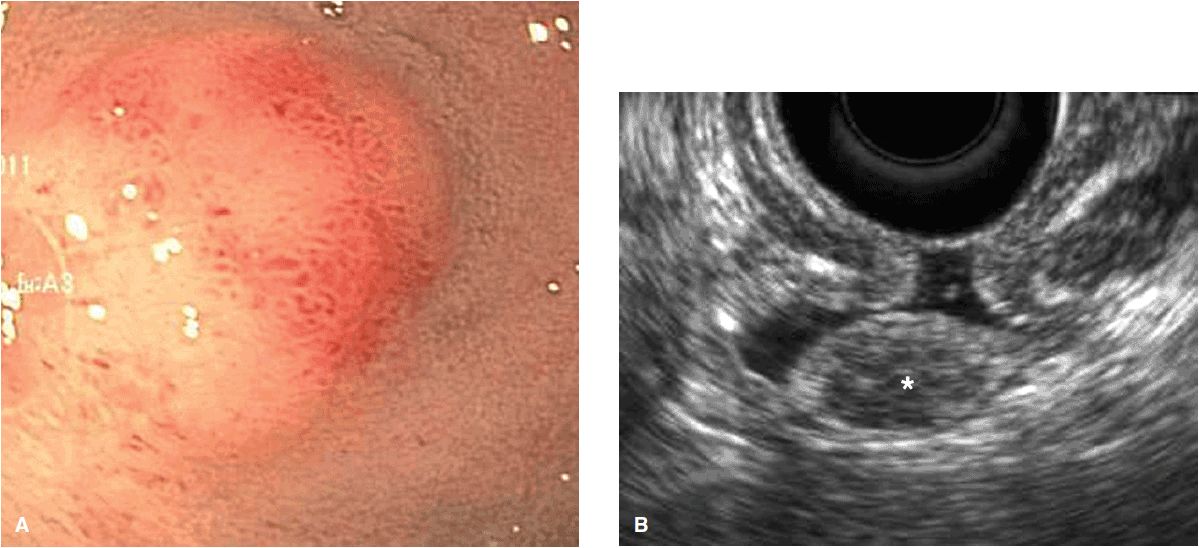
These lesions, oftentimes referred to as submucosal nodules, are bulges or masses that are visible on endoscopy from the GI lumen but that have overlying normal mucosa. The lesion can arise from within any of the intrinsic gastric wall layers or can be extrinsic to the GI wall causing an impression. Subepithelial lesions are most commonly determined to be mesenchymal tumors (54%) and include gastrointestinal stromal tumors (GISTs), leiomyomas, leiomyosarcomas, or schwannomas.31 While EUS alone cannot differentiate between these tumors, immunohistochemical staining performed on FNA samples can be diagnostic with a sensitivity and accuracy of 95% and 87%, respectively.32 Most GISTs stain positive for CD117 (c-kit) and CD 34, while leiomyomas express smooth muscle actin and desmin, and schwannomas are positive for S-100.33 While GISTs are the most common type of mesenchymal tumor and malignant in 20–25%,34 all GISTs are considered to have malignant potential. Endosonographic features can be used to help predict this potential risk for malignancy with sensitivities of 80–100%.35 – 37 For example, regularity of border, a homogenous pattern, and size <30 mm have been shown to be associated with benign GISTs, whereas necrotic centers and hyperechoic foci that suggest calcifications have been associated with malignant lesions (Figure 11-1A, 11-1B, and 11-1C).36,37 Additional subepithelial lesions include lipomas (5%), pancreatic rests (incidence 0.6–13.7%), carcinoid lesions, and cysts such as duplication cysts, granular cell tumors, inflammatory polyps, and varices. Gastric carcinoid is the most common type of neuroendocrine tumors (Figure 11-2). EUS provides the depth of invasion and vascularity of carcinoid tumors and can lead to an informed decision as to whether to attempt endoscopic resection.38 Extrinsic causes of subepithelial lesions include indentation by essentially any of the surrounding organs including spleen, splenic vessels, left liver lobe, gallbladder, and others. Extralumenal causes can easily be visualized by EUS.
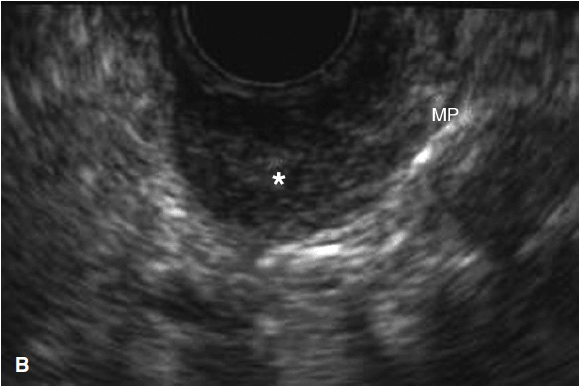
Figure 11-1. Endoscopic and EUS images of gastrointestinal stromal tumors (GIST). (A) Endoscopic view of submucosal nodule in duodenum; (B) EUS image of GIST (*) arising from muscularis propria (MP). The small size, Homogenous pattern and regular borders are consistent with benign histopathology; (C) EUS image of GIST of stomach wall with irregular borders, cystic component, large size, consistent with malignant histopathologic findings.
Figure 11-2. Carcinoid lesion of the duodenum. (A) Endoscopic view of submucosal lesion in duodenal bulb; (B) EUS image of the lesion characterized by defined margins, hypoechoic echogenicity, and arising from submucosal layer of duodenal wall.
 EUS of the Ampulla and Duodenum
EUS of the Ampulla and Duodenum
Benign ampullary lesions include adenomas, GISTs, lipomas, and neuroendocrine tumors, with an overall incidence of 0.04–0.21% of patients on autopsy studies.39 Ampullary adenomas are the most frequent of these and tend to follow an adenoma-carcinoma sequence, with 35–90% of adenocarcinomas arising from preexisting adenomas.40 Five-year survival ranges from 30–70% and is dependent on size and stage. Staging by EUS, based on the TNM classification system, has been demonstrated in a number of studies to have the greatest accuracy in T staging (78%) compared to CT (24%) and MRI (46%).41 Nodal staging has been shown to be equivalent, however, between EUS and CT.42,43
 EUS of the Pancreas
EUS of the Pancreas
Pancreatic malignancies
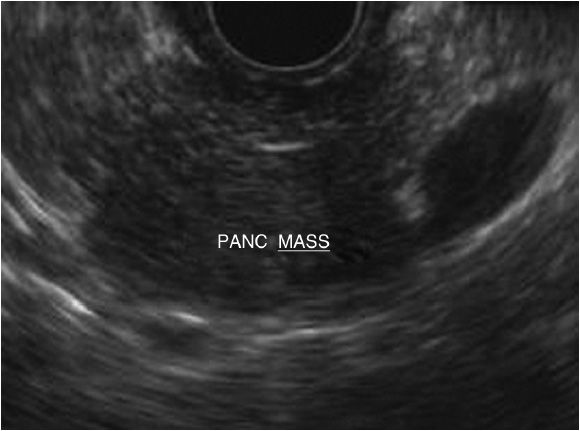
Pancreatic adenocarcinoma is the second leading cause of death among all GI cancers.44 Adenocarcinoma accounts for 90% of all pancreatic cancers.45 Additional malignancies include neuroendocrine tumors (NETs), exocrine tumors, lymphomas, and metastatic lesions.46 Early diagnosis of resectable lesions and the avoidance of unnecessary debilitating surgery are the key roles of the EUS for pancreatic cancer. Pancreatic tumors can often appear as a hypoechoic mass with irregular borders. If located in the head of the pancreas, it is can be associated with either pancreatic or biliary dilation or both (Figure 11-3). EUS allows for both accurate tumor staging, as well as real-time tissue sampling. EUS allows for thorough evaluation of vessel involvement such as portal vein, splenic vein, and arterial involvement, as well as the detection of ascites and possible metastases to the liver. Its superiority, compared to CT, has been demonstrated in pancreatic lesions <25 mm.47 The sensitivity and specificity of EUS and FNA is 85% and 98%, respectively, and has completely replaced pancreatic duct sampling during ERCP.47 In cases in which the diagnosis is indeterminate between inflammation and malignancy, fine needle biopsy (FNB) can be used. Fine needle biopsy has been shown to have up to 100% sensitivity in diagnosing autoimmune pancreatitis (AIP) in the setting of a pancreatic mass, as it provides greater amount of tissue to demonstrated specific histologic features needed to diagnosis this form of chronic pancreatitis.48,49
Figure 11-3. EUS image of pancreas mass. This lesion is described as hypoechoic with irregular borders arising within the body of the pancreas.
Pancreatic cysts
The incidence of pancreatic cyst detection is increasing with more frequent use of cross-sectional imaging studies such as CT and MRI. The most common cystic lesions are pseudocysts (80–90%), while cystic neoplasms, including serous cystadenomas (SCA), mucinous cystadenomas (MCN), mucinous cystadenocarcinomas (MCAC), and intraductal papillary mucinous neoplasm (IPMN), occur in 10% of cases.50,51 EUS not only can be used to help differentiate these lesions by morphological features, but can also provide fluid analysis via FNA to help determine malignant potential. Diagnosis based on morphology alone has an accuracy of 51–73%.52 The addition of FNA increases this accuracy to 79–94 %.52,53 Fluid analysis includes measurement of tumor markers such a CEA and amylase. In one prospective study, the optimal cutoff value of CEA level of 192 ng/mL, provided an accuracy of 79% to differentiate mucinous from nonmucinous cystic lesions.52 Elevated amylase levels provide additional confirmation that the cystic lesion is likely a pseudocyst or IPMN.54
Many factors have to be considered when discussing the surveillance of pancreatic cystic lesions with EUS, such as the age, comorbidities, and the malignant potential of the lesion. There has not been a universally accepted surveillance program to date due to lack of long-term studies with surgical and clinical follow-up. Cross-sectional imaging such as CT or MRI can be used for monitoring cyst and pancreatic duct size, leaving EUS with FNA for cysts that demonstrate increased risk for malignancy such as size >3 cm, mural nodules, or a solid component.55 Therapeutic indications for EUS in pancreatic cysts are mostly for the drainage of pancreatic pseudocysts via a transgastric or transduodenal approach. A retrospective study comparing surgical versus endoscopic management of noncomplicated pseudocysts found that both methods had equivocal efficacy; however, the endoscopic approach was more cost-effective and associated with a shorter length of hospital stay.56
Chronic pancreatitis
EUS has been used for over two decades for the diagnosis of chronic pancreatitis. The most current classification system revolves around the ability to identify characteristic endosonographic features commonly found in chronic pancreatitis. These criteria include but are not limited to hyperechoic duct wall, strands, nonshadowing hyperechoic foci, lobularity, cystic lesions, calcifications, and pancreatic duct irregularity.57 According to a consensus panel using a newer classification system (the Rosemont classification) the agreement on a final diagnosis of chronic pancreatitis was achieved in >90% of patients, with the highest accuracy being obtained in patients with a median number of four features on EUS.58 EUS can be used to evaluate pancreatic ductal dilation by ruling out underlying neoplasm and visualizing intraductal stones, thus identifying patients that may be amenable to pancreatic duct drainage via endoscopic retrograde pancreatography (ERCP).
 EUS of the Biliary System
EUS of the Biliary System
Bile duct cancer
The role of EUS in the staging of cholangiocarcinoma appears to be limited to determining nodal involvement and distant metastasis. The ability of EUS without FNA to differentiate benign from malignant disease has a sensitivity of 78% and specificity of 84%.59 FNA is typically performed to sample suspicious lymph nodes while FNA of potentially respectable lesions should be avoided.60 A safe technique to assess intraductal extension of a biliary cancer is intraductal ultrasonography (IDUS), which is performed during ERCP. IDUS has been reported to be superior to EUS for T staging cholangiocarcinoma and subsequent determination of resectability.61
Gallbladder cancer
Gallbladder carcinoma is the most common malignancy of the biliary tract, and is oftentimes found incidentally in cholecystectomy specimens. Unfortunately, when detected clinically, it is often diagnosed at an advanced stage when invasion into the liver and nodal dissemination has occurred.62,63 EUS can be used to distinguish T1 from T2 lesions. While T1 lesions can be managed by simple cholecystectomy, T2 lesions can be offered extended cholecystectomy (including resection of segments 4 and 5, with lymph node dissection), which is associated with a 90–100% 3-year survival rate.64,65 Additionally, EUS provides the opportunity to sample lymph nodes, liver metastases, and carcinomatous ascites to assist in staging of gallbladder carcinoma.66
Bile duct stones
Choledocholithiasis is found in 10–15% of patients undergoing cholecystectomy and can lead to severe complications such as cholangitis and/or pancreatitis.67 ERCP has become the favored therapeutic procedure to remove these stones; it has, however, been replaced by other modalities for diagnostic purposes due to associated procedure-related complications (namely pancreatitis) and its imperfect sensitivity (85–90%).68,69 Use of EUS prior to ERCP permits the avoidance of ERCP in approximately 50% of patients, thereby selecting the most appropriate patients for intervention.70 Although MRCP is a noninvasive option with an excellent accuracy for the detection of CBD stones (82–95%),71,72 EUS permits the detection of small stones impacted into the papilla due to improved spatial resolution and allows more thorough interrogation of the ampulla.73 In addition, there is no contraindication to performing EUS in patients with pacemakers or other devices that would otherwise prevent MRCP from being performed.
 Interventional EUS
Interventional EUS
The last decade has seen advancements in EUS instrumentation, which allows for improved visualization and access of previously unreachable targets and offers alternatives to percutaneous and surgical approaches. Interventional indications for EUS-guided access and drainage have been described in numerous reports for obstructed biliary systems, pancreatic ducts, and fluid collections.74–81 EUS-guided biliary drainage can be performed using a transgastric, transhepatic, or transduodenal-choledochal approach. The overall success rate was 84% with an overall complication rate of 16% in the largest series.77 EUS-guided pancreatography and drainage can be performed from a transgastric antegrade approach with performance of either a pancreaticogastrostomy or a pancreaticoduodenostomy. The largest series of this procedure reported a success rate of 92% with a complication rate of 6%.81 In the largest studies reported, a total of 368 patients underwent EUS-guided cystgastrostomy and cystduodenostomy with a global success rate of approximately 92% and a complication rate of 8%.82 Based on the available data, these advanced procedures, performed in expert hands, seem to offer safe and effective alternatives to radiological or surgical approaches. However, more data are required before there can be more widespread use of these techniques.
The future of EUS is yet to be determined, and may further bridge the endoscopic and surgical fields. Utilizing the access that is provided by therapeutic EUS, animal studies have been conducted to demonstrate its role in cutting-edge surgical techniques such as lymph node resection, cardiac biopsy, and the creation of gastrojejunal anastomosis.83
NORMAL ANATOMY/EUS APPEARANCE
Endoscopic examination of the GI tract is a dynamic examination that requires real-time determination and manipulation of the scope within the patient and uses landmarks to help identify normal and abnormal anatomy. Therefore, the anatomy and normal EUS appearance will be discussed in conjunction with scanning techniques in this chapter.
Gastrointestinal wall
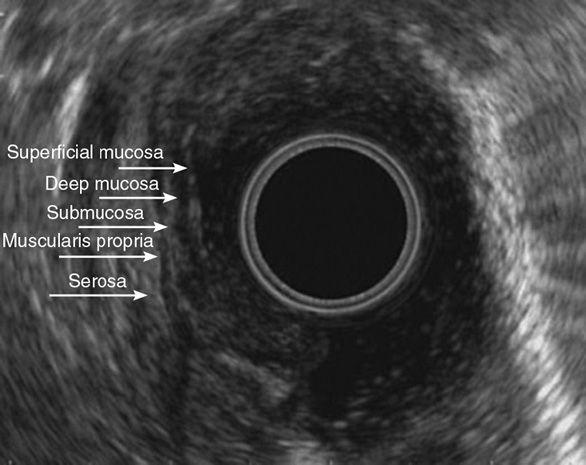
Visualization of wall layers is possible due to the immediate apposition of the transducer to the luminal wall. Inflation of the balloon on the transducer with water improves contact with the mucosa and this visualization significantly. The wall of the GI tract is divided into five distinct layers (Figure 11-4). The first layer visible from the echoendoscope is the interface between the mucosa and the transducer, which appears as a bright, hyperechoic structure. The second layer represents the deep mucosa or muscularis mucosa and appears hypoechoic. The third layer, which is hyperechoic, is the submucosa. The fourth layer, a hypoechoic layer, represents the muscularis propria. The fifth layer, a hyperechoic layer, represents the adventitia in the esophagus, and the serosa in the stomach and small bowel. The use of higher-frequency probes such as 20–30 MHz allows for additional differentiation of layers within the muscularis propia and the muscularis mucosa, increasing potential visualization to seven and nine layers, respectively.84,85
Mediastinum for distal esophagus, mid esophagus, and proximal esophagus
After visualization of the esophageal wall layers (Figure 11-5), EUS of the esophagus focuses on evaluating mediastinal structures. The parallel course of the esophagus to the longitudinal axis of the body allows for precise correlation of the mediastinum with transverse sections as seen on chest CT, using a radial echoendoscope. Early identification of the thoracic aorta with rotation of the scope so that it falls in the 5 o’clock position allows for orientation of the mediastinum to match cross-sectional imaging. When discussing images as seen with radial EUS, structures described on the right side of the screen will correspond to the patient’s right and those on the left to the patient’s left. Further description of the mediastinum can be divided by the location of the transducer into proximal, mid, and distal esophagus. The structures that can be visualized from the proximal esophagus include the left and right carotid arteries, left and right internal jugular veins, and bilateral lobes of the thyroid. These are not commonly evaluated during EUS and specific anatomical references and techniques are beyond the scope of this discussion.
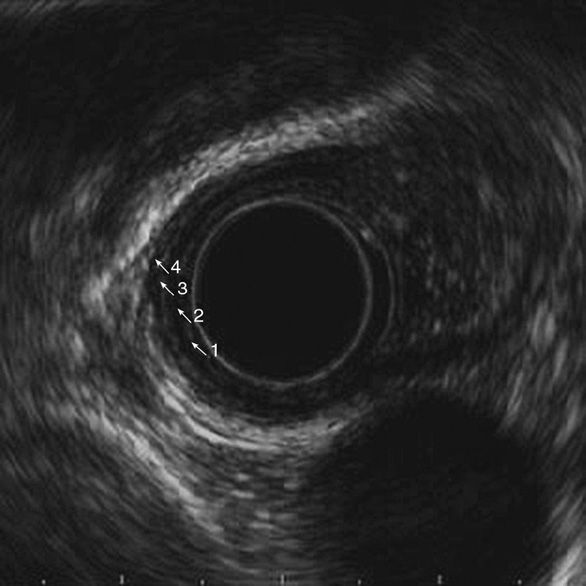
Figure 11-5. Normal esophageal wall layers. (1) Mucosa, hyperechoic; (2) muscularis mucosa, hypoechoic; (3) submucosa, hyperechoic; (4) muscularis propria, hypoechoic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree