Femoropopliteal occlusive disease is present in a significant proportion of older patients, with about 20% of men and 17% of women older than 55 living with ankle-brachial indices (ABI) under 0.9. A smaller percentage (3%-7%) present with symptoms of intermittent claudication (IC) between the ages of 60 and 70.1–5 After the age of 70, however, 25% of patients with IC clinically deteriorate, with 1% to 3.3% progressing to critical limb ischemia (CLI) and eventual amputation.6,7 Although only some patients experience worsening claudication or gangrene, many more adjust to this reduction in circulation by becoming progressively more sedentary. As such, the femoropopliteal arteries deserve respect as critical determinants in the health and mobility of our older citizens. To be sure, significant lower extremity occlusive arterial disease has ominous associations with generalized vascular disease, and the estimated 5-, 10-, and 15-year morbidity and mortality of these patients is 30%, 50%, and 70%, respectively, with 70% to 80% of deaths related to cardiovascular events (myocardial infarction, cerebrovascular accidents, and ruptured aneurysms). These figures are 2.5 times higher than that of nonclaudicants.2 The anatomy and physiology of the femoropopliteal artery is unique and poses specific challenges for both percutaneous and surgical treatments. The femoral artery is the longest artery in our body, with the fewest branches. It has thick muscular walls so that it can tolerate the variety of longitudinal and lateral compressive forces delivered to it by virtue of its fixation at the highly mobile hip and knee joints. In this milieu, catheter-based intervention often falters because this powerful artery scars, proliferates, and thromboses under the injuries inflicted by angioplasty, and it fractures and deforms metallic stents placed within its lumen.8 No wonder that multiple devices have tried and failed to tame occlusive disease in the femoropopliteal arteries. With almost 2000 articles pertaining to femoral artery stents available in the recent medical literature,8 the importance of critical manuscript review in this subject area cannot be understated. Therefore, the reader must consider important comparative data when evaluating the various treatments for femoropopliteal arterial disease—including, but not limited to, the status of the runoff and inflow vessels, the length and degree of calcification of lesions treated, clinical versus imaging follow-up, and the presence of concomitant disease states such as diabetes, continued smoking, or renal failure. Peripheral arterial disease (PAD) involving the lower extremities is defined as the presence of disease causing hemodynamic compromise resulting in a resting ABI of less than 0.9. The clinical presentation of PAD can range from no symptoms to gangrene and tissue loss. PAD is a strong marker for the presence of systemic atherosclerotic disease and is therefore associated with an increased risk of major cardiovascular events (5%-7% annually).2 The most common cause of occlusive disease in the femoropopliteal segment (FPS) is atherosclerosis, with the major risk factors presented in Table 36-1 along with targets for reduction and the means for reaching those targets. TABLE 36-1 Risk Factors for Peripheral Vascular Disease As discussed earlier, many factors contribute to the development and progression of atherosclerosis (see Table 36-1). Attempts should be made to address all modifiable risk factors. Surprisingly, medical therapies have not been instituted or optimized in a large proportion of patients referred for lower extremity revascularization. This represents an opportunity and an obligation for the interventionalist to evaluate and institute appropriate therapies for various risk factors. Along with efforts to modify a patient’s risk factors, a program of structured exercise is the recommended initial step in the treatment of IC. Patients with CLI, however, bypass this step and generally proceed directly to revascularization owing to their advanced occlusive disease and walking impairment. Supervised exercise conducted for 3 months or longer increases treadmill performance and lessens the severity of claudication,9 but there are three major limitations to exercise programs. First, unfortunately, exercise is contraindicated in 9% to 34% of PAD patients, such as those with severe coronary artery disease or neurologic and musculoskeletal impairment. Second, PAD patients are usually poorly compliant with exercise programs, which are furthermore not widely available. Finally, and even worse, these exercise programs are not reimbursed by most insurance companies even though they are proven to be cost-effective compared to surgical or endovascular vascular procedures.10,11 Structured exercise programs are therefore not widely prescribed. Many pharmacologic agents have been studied for the relief of symptoms of IC. Only a few, however, have shown any evidence of clinical utility, and they do not provide the same level of symptom relief as a successful revascularization.12 The first U.S. Food and Drug Administration (FDA) agent approved for claudication was pentoxifylline, a methylxanthine derivative thought to increase red blood cell membrane deformability and decrease fibrinogen activity and platelet adhesion, with the overall effect of reduced blood viscosity. Multiple trials have failed to show other than mild clinical benefit, so this drug is probably beneficial only to those who cannot tolerate cilostazol,13 a better claudication drug. Cilostazol is a phosphodiesterase-III inhibitor with some antiplatelet and vasodilatation activity. As such it should not be prescribed to patients with congestive heart failure (CHF), owing to decreased survival compared to placebo in patients with class III-IV CHF. Cilostazol increases maximal and pain-free walking distances by half and two thirds, respectively, after 3 to 6 months of therapy,14 but its side effects of diarrhea, headaches, and dizziness present a compliance issue, with up to 60% of patients stopping the drug within 3 years.15 Use of antiplatelet agents, specifically aspirin, is an important topic and deserves more attention. Aspirin use has been associated with a 25% odds reduction in subsequent cardiovascular events in patients with cardiovascular disease.16 Although an initial meta-analysis by the Antithrombotic Trialists’ Collaboration concluded aspirin use in patients without any evidence of vascular disease does not statistically reduce cardiovascular events,17 more recent studies combining data from trials using not only aspirin but also other antiplatelet agents showed a significant reduction in ischemic events in patients with PAD. The American College of Cardiology and American Heart Association (ACC/AHA) recommend aspirin or clopidogrel daily for cardiovascular risk reduction in patients with PAD, and studies do indicate a relative risk reduction in all cardiovascular events for patients receiving such agents, especially clopidogrel.18 Other agents such as naftidrofuryl, propionyl-l-carnitine, and some lipid-lowering agents have also shown promise in improving walking distance in patients with IC. Bypass surgery has been the traditional treatment modality in the majority of patients in need of lower extremity revascularization, but the less invasive nature of endovascular approaches and better outcomes data are shifting this paradigm. The change was reflected in the 2007 TransAtlantic Inter-Society Consensus (TASC II) document that expanded recommendations for endovascular therapies.2 TASC II classification of diseases of the FPS is shown in Table 36-2. Although TASC class A and B lesions in symptomatic patients are best treated endovascularly, the recommended treatment for class D patients is surgical bypass in suitable candidates. Class C patients represent the “gray area” where endovascular techniques are applicable in selected patients not suitable or agreeable to surgical bypass. These recommendations are based on the consensus of a panel of experts after reviewing the current literature. For comparison purposes, the surgical gold standard for treating the diseased FPS is femoral-to–above knee popliteal venous bypass with 6-month, 1-, 2-, 3-, and 4-year primary patency rates of 87%, 81%, 77%, 71%, and 70%, respectively, with these numbers slightly decreasing when prosthetic material is used as the bypass conduit (85%, 77%, 66%, 59%, and 51% at the same time intervals).19 TABLE 36-2 TASC II Classification of Femoropopliteal Segment Disease Adapted from Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:1S–68S. The remainder of this chapter describes current endovascular approaches to femoropopliteal occlusive disease. Table 36-3 lists patency rates, complications, and the lesion types and locations suitable for various techniques as described in the recent literature. These data are best compared and contrasted with the results from best medical therapy, surgical bypass, and balloon angioplasty (also included, where possible, in Table 36-3). Even the most modern endovascular devices marketed for the FPS (and there have been many) struggle to achieve results comparable to balloon angioplasty, and most remain woefully inferior to surgical bypass. We will briefly describe those devices falling short of expectations, then conclude with a discussion of the best currently available techniques to reliably open diseased femoropopliteal arteries—namely nitinol stents and stent-grafts. TABLE 36-3 Comparison of Current Treatment Methods for Superficial Femoral Artery Occlusive Disease
Endovascular Management of Chronic Femoropopliteal Disease
Clinical Presentation
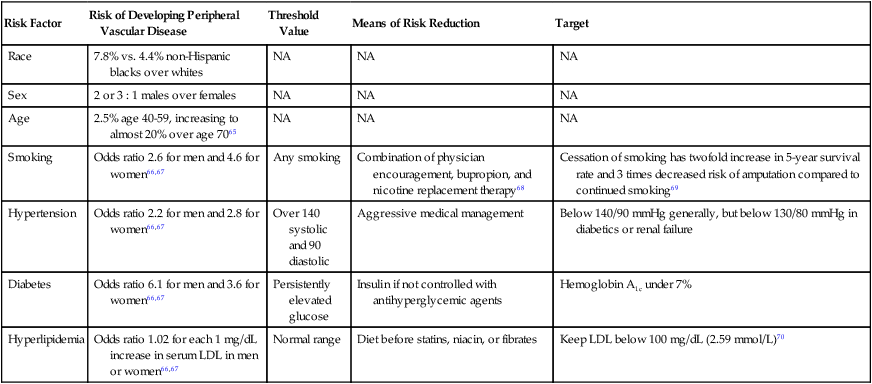
Risk Factor
Risk of Developing Peripheral Vascular Disease
Threshold Value
Means of Risk Reduction
Target
Race
7.8% vs. 4.4% non-Hispanic blacks over whites
NA
NA
NA
Sex
2 or 3 : 1 males over females
NA
NA
NA
Age
2.5% age 40-59, increasing to almost 20% over age 7065
NA
NA
NA
Smoking
Odds ratio 2.6 for men and 4.6 for women66,67
Any smoking
Combination of physician encouragement, bupropion, and nicotine replacement therapy68
Cessation of smoking has twofold increase in 5-year survival rate and 3 times decreased risk of amputation compared to continued smoking69
Hypertension
Odds ratio 2.2 for men and 2.8 for women66,67
Over 140 systolic and 90 diastolic
Aggressive medical management
Below 140/90 mmHg generally, but below 130/80 mmHg in diabetics or renal failure
Diabetes
Odds ratio 6.1 for men and 3.6 for women66,67
Persistently elevated glucose
Insulin if not controlled with antihyperglycemic agents
Hemoglobin A1c under 7%
Hyperlipidemia
Odds ratio 1.02 for each 1 mg/dL increase in serum LDL in men or women66,67
Normal range
Diet before statins, niacin, or fibrates
Keep LDL below 100 mg/dL (2.59 mmol/L)70
Treatment
Medical Management
Surgical Revascularization Procedures
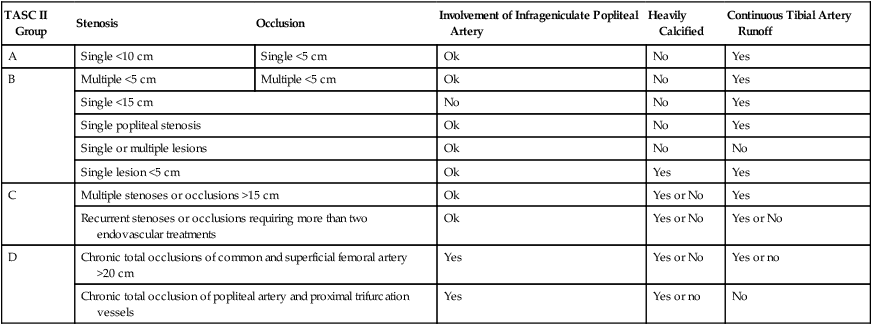
TASC II Group
Stenosis
Occlusion
Involvement of Infrageniculate Popliteal Artery
Heavily Calcified
Continuous Tibial Artery Runoff
A
Single <10 cm
Single <5 cm
Ok
No
Yes
B
Multiple <5 cm
Multiple <5 cm
Ok
No
Yes
Single <15 cm
No
No
Yes
Single popliteal stenosis
Ok
No
Yes
Single or multiple lesions
Ok
No
No
Single lesion <5 cm
Ok
Yes
Yes
C
Multiple stenoses or occlusions >15 cm
Ok
Yes or No
Yes
Recurrent stenoses or occlusions requiring more than two endovascular treatments
Ok
Yes or No
Yes or No
D
Chronic total occlusions of common and superficial femoral artery >20 cm
Yes
Yes or No
Yes or no
Chronic total occlusion of popliteal artery and proximal trifurcation vessels
Yes
Yes or no
No
Treatment
Initial Success (%)
Primary Patency (%)
Secondary Patency (%)
Adjunct Use of Stents or Angioplasty (%)
Restenosis on Ultrasound (%)
6 mo
1 yr
Longer (time)
6 mo
1 yr
Longer (time)
Venous above-knee femoropopliteal bypass
87
81
77 (3 yr)
Prosthetic above-knee femoropopliteal bypass
85
77
66 (3 yr)
Balloon angioplasty
95
63
58
55-68 (3 yr)
Atherectomy (SilverHawk)
62
52 (18 mo)
80.3
75 (18 mo)
35
Atherectomy (Pathway)
99
73
50
30 (6 mo) 40 (1 yr)
Laser (Spectranetics)
90.5
59
54
75
88
41 (6 mo) 46 (1 yr)
Cryoplasty
94
63
55
91
91
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access






