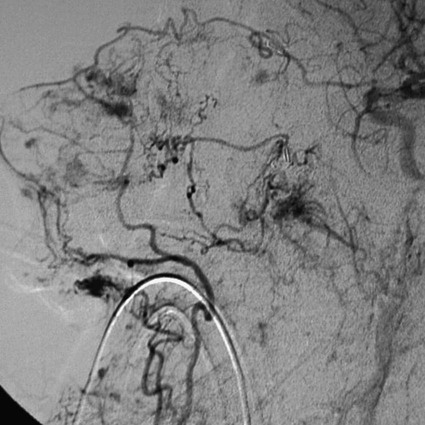
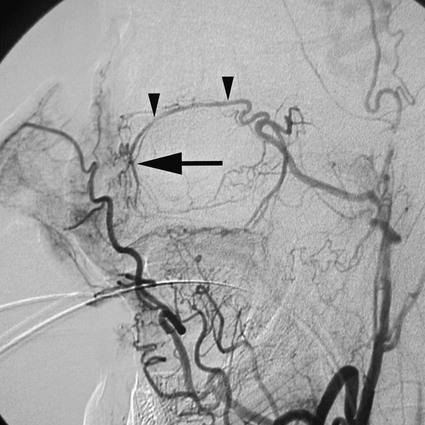
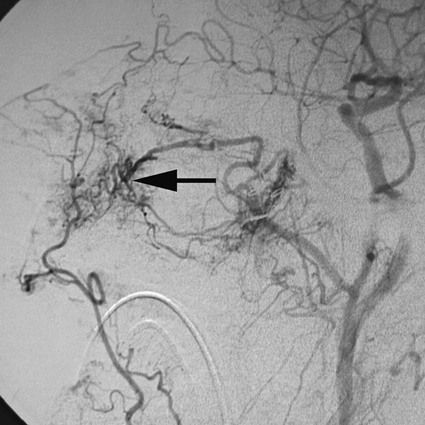
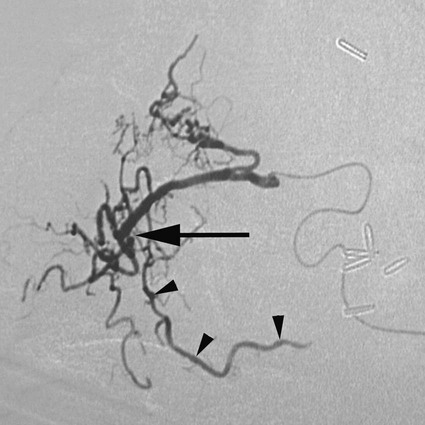
Rush H. Chewning, Gerald M. Wyse, Philippe Gailloud and Kieran P.J. Murphy Local conservative treatments are first line in managing epistaxis. A common treatment is insertion of pledgets soaked in an anesthetic vasoconstrictor solution, along with pressure on both sides of the nose.1 If this or another similar local treatment fails to stop the bleeding, the nose is typically packed, from posterior to anterior, with gauze or a preformed nasal tampon (Merocel or Doyle sponge). To pack the nose, a catheter is inserted through the nostril and nasopharynx and out the mouth. A gauze pack is then attached to the end of the catheter and positioned in the posterior nasopharynx to seal the region and compress the site of bleeding.1 Complications of nasal packing procedures include septal hematomas and abscesses from traumatic packing, sinusitis, and pressure necrosis secondary to excessively tight packing. Though rare, major complications such as septicemia, arrhythmia, hypoxia, and death have also been associated with packing.2,3 Use of an epistaxis balloon is an alternative method of tamponade. For anterior bleeding, balloons are inserted along the floor of the nasal cavity and inflated slowly with sterile water or air until the bleeding stops. For posterior epistaxis, a double-balloon device is passed into the nasopharynx and seated in the posterior nasal cavity to tamponade the bleeding source. Next, the anterior balloon is inflated in the anterior nasal cavity to prevent retrograde travel of the posterior balloon and subsequent airway obstruction. If a specialized epistaxis balloon is unavailable, a Foley catheter may be substituted using the same general principles. Balloon tamponade is associated with a risk of saline aspiration,4 pressure necrosis, perforation, infection, and other potential complications of packing. Persistent bleeding requires endoscopic inspection by an ear, nose, and throat (ENT) specialist to identify the bleeding site. Cautery is first-line management if an anterior source of bleeding has been visualized.5 However, cautery will not take effect through fluid, so the bleeding point itself cannot be cauterized until hemostasis is achieved. Cautery may cause rhinorrhea and crusting of the mucosa; overzealous use of the technique could lead to ulceration and perforation.6 For intractable epistaxis, surgical ligation or endoscopic cauterization of the internal maxillary, external carotid, or sphenopalatine artery may be considered. Potential complications of such procedures include excessive blood loss, periorbital infection, and paresthesia. Rare major complications include oculomotor paralysis, oroantral fistulas, and septal perforation.7 Angiographic embolization in the case of intractable epistaxis has become an increasingly common management approach. The first successful percutaneous embolization for epistaxis was reported by Sokoloff et al. in 1974.8 The technique was applied to patients with hereditary hemorrhagic telangiectasia (HHT) by Strother and Newton in 1976, and in 1980, Merland et al. were able to report a 97% success rate in a first series of 54 patients with severe epistaxis.9,10 The technique has since been widely accepted and its efficacy confirmed.11 Embolization may be considered a safe and effective therapy for epistaxis.12 In addition, it is particularly helpful in cases where ENT surgery has failed to stop bleeding, usually after an endoscopic approach. Although endovascular treatment of epistaxis is safe, serious complications are still possible. Tseng et al. reported 2 cases of stroke in 114 patients treated,11 Elden et al. noted 1 stroke in 108 patients,12 and Remonda et al. reported 2 strokes in 47 patients treated endovascularly for severe epistaxis.13 With the use of calibrated particles, complications such as soft-tissue necrosis and facial paralysis have also been noted.14 Complications related to coils and Gelfoam include temporofacial pain and headache.15 The nasal mucosa receives its blood supply principally from the maxillary artery via the sphenopalatine artery and its branches. Patients with intractable epistaxis commonly show diffuse bilateral mucosal anomalies on catheter angiography (Fig. 95-1). It is therefore not surprising that endovascular management of epistaxis has classically involved bilateral sphenopalatine and sometimes bilateral facial artery angiography, followed by targeted embolization of offending vessel branches with various embolic materials such as calibrated microparticles and Gelfoam pledgets.15,16 Although the collateral network connecting the sphenopalatine artery and its branches to the facial artery and the anterior and posterior ethmoidal arteries is well recognized,17 the possibility of mucosal blood supply from branches of the infraorbital artery has not been previously emphasized. In rare cases, the infraorbital artery should be considered a possible source of bleeding and a potential candidate for superselective embolization in patients with intractable epistaxis18 (Fig. 95-2). In addition to diffuse mucosal changes, a focal arterial anomaly can often be detected (Fig. 95-3). The combination of a bleeding source suspected clinically and a focal anomaly seen on angiography allows precise targeting of embolization to a single vessel, despite the presence of widespread mucosal hypervascularity. This strategy, which relies on close collaboration between the otorhinolaryngologist and the interventional neuroradiologist, minimizes the potential for periprocedural complications and recurrent hemorrhage. The use of n-butyl cyanoacrylate (NBCA) glue as an agent in the treatment of epistaxis has been documented. Merland et al. used Gelfoam occasionally supplemented by NBCA glue at the end of the procedure.10 Luo et al. reported using NBCA glue to treat pseudoaneurysms associated with hypervascular tumors of the head and neck.19 NBCA glue as the primary embolic agent for treatment of severe epistaxis was first documented by Gailloud et al.18 (Figs. 95-4 and 95-5).
Endovascular Management of Epistaxis
Treatment Options
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine