4 Endovascular Treatment of Craniocerebral Diseases
Contents
Treatment of AV Malformations (Angiomas)
Dural AV Fistulas (Dural Fistulas)
Treatment of Cerebral Aneurysms
Pathology and Treatment Principles
Preoperative Devascularization of Hypervascular Tumors
Juvenile Nasopharyngeal Fibromas
Thrombosis, Thrombolysis, and Thrombolytic Agents
Indications and Contraindications
Previous Experience and Results
Treatment Principles
The safe and effective endovascular treatment of craniocerebral diseases requires an angiography system (digital subtraction angiography [DSA]) with a 512 × 512 or higher image matrix. The capacity for simultaneous operation in two orthogonal planes is a desirable feature.

Endovascular therapy always starts with angiographic mapping of the vessels.
The use of sedation or general anesthesia during the procedure depends on the nature of the disease and the preference of the operator. First a catheter sheath with an outer diameter or 4–6 F is placed percutaneously in the femoral artery. A sheath makes it easier to exchange the diagnostic catheter for the outer catheter and coaxial selective catheter that will be used for the actual intervention.

The results of diagnostic angiography determine the type of catheter and embolic material that should be used for selective catheterization.
Sometimes it is expeditious to use separate approaches for the diagnostic catheter and the interventional outer catheter (e.g., both femoral arteries or one femoral artery and a brachial artery).
The endovascular route to an intracranial vascular lesion can be long and winding. Even a normal cerebral angiogram shows many physiologic curves, and the vessels leading to an AV malformation, for example, often show additional flow-induced curves and tortuosity. If the lesion is located far distally, close to the brain surface, it will be necessary to negotiate a particularly long endovascular pathway. Correspondingly high demands are placed on the catheterization technique and the materials that are used. The catheter system should not irritate the access vessels, nor should it occlude the proximal part of the vessel. Also, the system should allow for fast catheter removal after the embolization, especially when glue has been deposited.
Catheterization Techniques
Catheterization techniques have evolved from direct puncture of the carotid or axillary artery to the transfemoral approach which is favored today. A number of catheterization systems have proved effective for selective catheterization and embolization, and a variety of configurations are used for different vascular morphologies and flow velocities. The systems generally consist of a preformed outer catheter with high torque stability, which is advanced into a large supra-aortic vessel. Next a coaxial selective catheter is threaded through the outer catheter. The selective catheter consists of a proximal shaft, preferably with high torque stability, on which a thinner, soft, distal catheter segment is mounted.

Pressure irrigation is maintained between the outer and inner catheters to prevent thrombus formation within the coaxial system.
Regardless of the catheter configuration and interventional approach, current systems employ one of two strategies for delivering the catheter to the intended target: guidewire-directed catheterization and flow-directed catheterization.
 Guidewire-Directed Catheterization
Guidewire-Directed Catheterization
A prime example of this technique is the Tracker catheter (Target Therapeutics), which comes in various lengths and configurations. After the outer catheter has been placed in the desired supra-aortic vessel, the coaxial selective catheter is advanced to the intervention site with the aid of guidewires, which are available in various materials and configurations.
 Flow-Directed Catheterization
Flow-Directed Catheterization
In this technique the blood flow is used to transport the catheter into the desired vessel. A good example is the Magic catheter (Bait). Flow-directed systems employ very soft, thin catheters, but like other catheters they can be manipulated using special guidewires. After the outer catheter has been introduced by the transfemoral route, a coaxial microcatheter is advanced through it. The extremely soft distal end of the inner catheter has a different diameter and a radiopaque marker at the tip. The variable flare of the catheter tip enables the system to be carried into the desired vasculature by flowing blood. The more proximal part of the catheter is long and rigid with high torque stability. With this design, the catheter can be advanced manually until it emerges from the end of the outer catheter. With the catheter tip thus deployed, the entire system can be carried distally by the blood flow along a tortuous path.
Embolic Materials
Table 4.1 lists the embolic materials that are used in modern endovascular procedures.
 Balloons
Balloons
Serbinenko and Debrun were the first, in the early 1970s, to describe the use of balloon catheters for the endovascular treatment of craniocerebral lesions. These catheter systems consist of latex balloons that are tied with elastic ligatures to a thin selective catheter. After maneuvering the balloon into position and inflating it to the desired size with contrast material or a polymerizing agent, the operator pulls on the catheter to detach it from the balloon. As the catheter is withdrawn, the ligature tightens to form a leakproof seal around the neck of the balloon. This type of balloon catheter is still used today for some indications, but in commercially available systems the ligature has been replaced by a valve system in the neck of the balloon.
Nondetachable microballoons have been described as an alternative to detachable balloons. They are mounted on microcatheters with at least a 1-F outer diameter. The balloons are secured in such a way that detachment cannot occur without destroying the entire system. Nondetachable balloons are used mainly for temporary occlusions, usually of larger vessels. They can also be used for the definitive occlusion of vessels or AV fistulas by permanently implanting the balloon and the microcatheter into the vascular system.
| Particles: |
| • PVA (Ivalon, Contour) |
| • Gelatin sponge (Gelfoam) |
| • Lyophilized dura |
| Tissue adhesives: |
| • Ethibloc |
| • Histoacryl |
| • Bucrylate |
| Balloons: |
| • Detachable |
| • Nondetachable |
| Other materials: |
| • Tantalum |
| • Lipiodol |
| Coils: |
| • “Free” coils |
| • Electrically detachable coils |
| • Mechanically detachable coils |
| • Laser-detachable coils |
| Vasculitis- or thrombosis-inducing agents: |
| • Absolute alcohol |
| • Thrombase |
 Tissue Adhesives
Tissue Adhesives
Cyanoacrylate adhesives (glues) were also introduced in the 1970s for the occlusion of cerebral arteriovascular (AV) malformations and AV fistulas, based largely on the work of Kerber. Cyanoacrylate adhesives polymerize on contact with ions. When injected undiluted, they harden in less than 1 s. This has several disadvantages for therapeutic use.
Disadvantages of cyanoacrylate adhesives
 High risk of catheter gluing
High risk of catheter gluing
 The injected vessel is usually occluded, but the arterial-to-venous connection usually remains patent
The injected vessel is usually occluded, but the arterial-to-venous connection usually remains patent
Consequently, diluents are added to the glue to adapt the polymerization to individual circumstances, and the catheter is flushed with 5 % glucose solution to expel free ions and keep the glue from polymerizing while still in the catheter. Oily contrast material is mixed with the glue to prolong the polymerization time. The visibility of the embolic agent during and after the injection can be enhanced by adding tantalum powder. Today, cyanoacrylate glues are widely used as embolic agents in the endovascular treatment of cerebral AV malformations and AV fistulas.
 Microparticles
Microparticles
Various particles have been used for the mechanical occlusion of vessels, especially small-caliber tumor vessels. All of these particles have embolic properties, regardless of their chemical composition. Particles made of polyvinyl alcohol (PVA) are the most widely used and are available in various sizes.
 Coils
Coils
Vessels can be occluded with metal coils made of platinum, tungsten, or other noble metals and supplied in a variety of lengths and diameters. A delivery wire (“coil pusher”) is used to advance the coil through the microcatheter to the desired intravascular site. Two basic types of coil are used.
Types of embolic coils
 Free coils are not rigidly attached to a delivery wire
Free coils are not rigidly attached to a delivery wire
 Detachable coils are released from the delivery wire by a mechanical, electrolytic, or laser-triggered mechanism
Detachable coils are released from the delivery wire by a mechanical, electrolytic, or laser-triggered mechanism
Some coils are woven with small Dacron fibers to enhance their thrombogenicity. After the coils are deployed from the tip of the microcatheter, they assume their original shape and expand against the vessel wall. When the coil has been correctly positioned, it is detached.
Treatment of AV Malformations (Angiomas)
Pathology and Pathophysiology
AV malformations (AVMs) are also sometimes referred to as “angiomas,” but it is better to avoid that term because of its tumor-like connotation.

AVMs are congenital vascular malformations composed of numerous microscopic shunts between arteries and veins.
The AVM is fed by enlarged, misshapen arteries and drained by similarly abnormal veins. The entire tangle of feeding and draining vessels and AV connections is called the nidus, based on its appearance at operation. The enlargement of the nidus that occurs in untreated cases does not represent actual growth but is a passive, pressure-induced dilatation of the draining veins and arterial feeders caused by the increasing flow volume across the lesion. AVMs are congenital, but most do not become symptomatic until early adulthood. The following four symptom complexes may occur:
• Single or recurrent intracranial hemorrhages from the rupture of arterialized veins,
• Progressive neurologic deficits caused by deficient blood flow to brain areas distal to the AVM,
• Epileptic seizures,
• Migraines or simple headaches, often unilateral.
Besides symptomatic AVMs, the more widespread use of computed tomography (CT) and magnetic resonance imaging (MRI) has resulted in the more frequent detection of asymptomatic AVMs.
Several studies have been done on the natural history of untreated AVMs. According to these studies, the risk of suffering an intracranial hemorrhage from a previously asymptomatic AVM is 1–5% per year.

Each hemorrhage doubles the risk that a subsequent hemorrhage will occur. Apparently, however, the risk declines again after about 10 years.
Endovascular Therapy
The embolization of intracranial angiomas may be done as a curative procedure or as a prelude to surgery or radiotherapy, depending on the situation. Initial efforts to occlude cerebral AVMs by embolization were undertaken in 1960 by Luessenhop (who instilled particles into the extracranial carotid artery). The development of superselective catheters and polymerizing embolic agents during the 1970s rendered this technique of nonselective embolization obsolete.
Today the transfemoral approach has become standard for the endovascular treatment of AVMs. Following selective catheter placement near the AVM nidus, angiography confirms that the embolization will spare the arteries that supply the brain. A more certain method is the Wada test, which involves the injection of an extremely short-acting barbiturate (Amobarbital, Amytal). The drug causes a very brief functional deficit in the cerebral territory of the injected vessel. When it is certain that the embolization will produce the desired result, the nidus of the AVM is occluded.
Various types of embolic material have been described in the literature, namely:
• Polymerizing agents such as Histoacryl and Bucrylate,
• Precipitating agents such as Ethibloc,
• PVA particles,
• Coils.

The goal of embolization is to permanently occlude the nidus and its AV connections. If only the arterial feeders are occluded, the lesion might become revascularized through collateral channels.
The veins should be left patent if at all possible. The agents most likely to effect permanent AVM occlusion are cyanoacrylates such as Histoacryl and Bucrylate, provided the material polymerizes in the nidus. Ethibloc also seems to afford permanent occlusion, even though the embolic material is absorbed over time. AVMs that have been occluded with particles appear to be the most likely to reopen. The same applies to AVMs whose feeders have been occluded with platinum coils.
Most AVMs require a staged embolization in several sessions. The rare lesions that are fed by a singe artery are an exception to this rule. For ordinary AVMs that are supplied by two or more arteries, the nidus and shunt volume can be reduced in a staged fashion. Nevertheless, the complete endovascular occlusion of AVMs is achieved in only about 10-30% of patients. The reasons for this relate to vascular architecture and catheterization techniques are:
• Marked tortuosity of the access vessel,
• Progressive reduction of flow velocity in the vessel,
• Distal location of the AVM.
The progressive flow reduction often poses an obstacle to further embolizations, especially when flow-directed catheters are used. If the goal of treatment is to eliminate the risk of hemorrhage, the endovascular procedure should be followed by additional treatments that focus on the residual portion of the AVM that cannot be embolized. The subsequent treatment generally consists of neurosurgical removal of the residual AVM (Fig. 4.1). Another option is stereotactic irradiation (“radiosurgery”).
Patients with a progressive neurologic deficit caused by a steal effect will benefit from partial endovascular obliteration of the AVM. Unfortunately, though, this treatment is unlikely to reduce the risk of hemorrhage to a significant degree. Endovascular procedures can also improve an AVM-induced seizure disorder and may even completely eliminate the seizures and the need for medication. In our experience, this can be accomplished more often than is commonly believed.
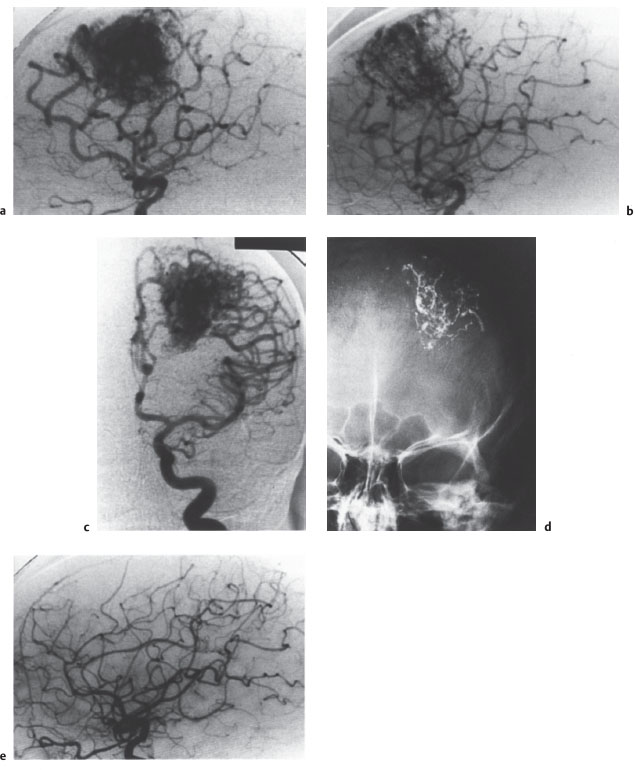
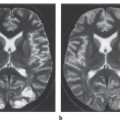
Fig.4.1a-e Combined endovascular and neurosurgical elimination of a frontal AVM.
a, b Left carotid angiograms show the pretreatment appearance of the AVM in the lateral (a) and AP projection (b).
c Following treatment by the superselective injection of Histoacryl in a total of three sessions, approximately 70 % of the AVM has been occluded. The presence of perforating feeders and arterioarterial anastomoses precluded any further embolizations.
d The embolic material appears as a spidery structure on an AP radiograph of the skull.
e Lateral angiogram after neurosurgical removal of the residual AVM shows a normal vascular pattern. The young woman tolerated both procedures without complications and is free of symptoms.
 Complications of Endovascular Therapy
Complications of Endovascular Therapy
The most serious complication of endovascular therapy is hemorrhage resulting from selective cerebrovascular catheterization or embolization. The major causes of postinterventional hemorrhage are listed in Table 4.2.
A review of the literature (1063 patients) shows that the average risk of hemorrhage is 6.8%: 7.4% with isobutyl cyanoacrylate (IBCA), 8.9% with Histoacryl, and 1.3 % with nonselective particle deposition in the carotid artery.

It appears that all embolic materials that have been used to date are capable of causing cerebral hemorrhage.
In slightly less than 50% of patients, subarachnoid or intracerebral hemorrhage occurs during the embolization. Nearly 50% of patients die from the hemorrhage, and more than 20% sustain permanent neurologic deficits. The rest recover without sequelae.
Patient Selection
Endovascular therapy seldom provides the complete obliteration of an AVM, and so it rarely eliminates the risk of hemorrhage. For this reason, its use must be justified by reducing the morbidity and mortality of a subsequent therapeutic procedure—usually neurosurgical resection or in some cases stereotactic irradiation of the residual AVM.
| • Normal pressure breakthrough (Spetzler) |
| • Stump rupture (Hassler) |
| • Patent AV connection, occluded vein |
| • Vessel wall necrosis caused by the embolic agent |
| • Injection pressure during the embolization |
| • Hemorrhage present during the embolization |

Endovascular therapy is appropriate as a solitary treatment if complete obliteration of the AVM appears feasible and the spontaneous course of the lesion would pose a greater cumulative risk to the patient than the endovascular procedure.
Endovascular therapy combined with neurosurgical resection of the AVM is appropriate if neurosurgery alone would carry a higher risk than if it were preceded by partial endovascular occlusion.
Active treatment is not indicated if there is no reasonable prospect of completely obliterating the AVM and the therapeutic risk outweighs the risk of leaving the lesion alone.
Neurosurgery alone is a sound option if the surgical risk is low. But this is true only if the treatment goal is to prevent hemorrhage. In patients with hemodynamically determined neurologic deficits, even partial endovascular obliteration of the AVM will lead to a significant improvement in symptoms.
Carotid—Cavernous Fistula
Fistulas between the internal carotid artery and cavernous sinus are classified into three groups based on pathogenic and hemodynamic criteria.
Classification of fistulas
 Direct traumatic carotid–cavernous fistula
Direct traumatic carotid–cavernous fistula
 Direct spontaneous carotid–cavernous fistula
Direct spontaneous carotid–cavernous fistula
 Indirect dural carotid–cavernous fistula
Indirect dural carotid–cavernous fistula
In the first two forms, a direct AV connection is established between the internal carotid artery and cavernous sinus either as a result of head trauma causing rupture of the cavernous segment of the artery or due to the rupture of a preexisting intracavernous aneurysm. Both types of fistula are characterized hemodynamically by high flow.
Indirect carotid—cavernous fistulas have an unknown pathogenesis. They are supplied by minimally dilated dural branches of the external or internal carotid artery and have relatively low flow. Due to the favorable collateral pathways along the dura, this type of fistula can receive flow from both the ipsilateral and contralateral carotid arteries.
 Direct Carotid—Cavernous Fistulas
Direct Carotid—Cavernous Fistulas
Spontaneous and posttraumatic direct carotid—cavernous fistulas are clinically indistinguishable, but the history can provide important clues. In both cases, the following signs are noted on the side of the fistula:
• Pulsatile exophthalmos
• Chemosis
• Pulsatile tinnitus
• Impaired ocular motility.
The first endovascular procedure that was tried in the treatment of carotid—cavernous fistula consisted of occluding the internal carotid artery with a Fogarty balloon catheter. When good collateral flow is present, occlusion of the carotid artery at the fistula is a low-risk, definitive treatment that is still used today in cases where selective forms of treatment have failed. Meanwhile, the development of detachable mini-balloons has provided an elective option that enables the permanent occlusion of carotid—cavernous fistulas while preserving the carotid lumen.
The technique currently used for the endovascular occlusion of a carotid—cavernous fistula is as follows: Once the cerebral blood supply has been mapped by angiography, the guide catheter is placed in the internal carotid artery on the affected side via a transfemoral approach. A coaxial balloon catheter is then passed through the outer catheter, and the high flow from the arterial side is used to direct the partially inflated balloon across the fistula into the cavernous sinus. The balloon is then inflated further, occluding the fistula from the venous side (Fig. 4.2). Several balloons may be necessary to occlude large carotid—cavernous fistulas.
In approximately 50% of patients, this technique is successful in occluding the fistula while preserving internal carotid arterial flow to the brain. Scarring across the orifice of the fistula maintains the occlusion, despite the tendency of the balloon to lose pressure and shrink during the first 2 weeks after the procedure. In the remaining 50% of cases, it used to be necessary to occlude the internal carotid artery at the level of the fistula. Today, however, new materials often permit the use of an elective procedure. Even coils can now be used successfully for carotid—cavernous occlusions. Also, a transvenous approach can be used instead of the arterial route. In this technique the cavernous sinus is catheterized via the femoral vein, inferior vena cava, superior vena cava, internal jugular vein, and inferior petrosal sinus. For hemodynamic reasons, however, the venous approach cannot be used for balloon embolizations. Since a recurrence of the fistula produces very characteristic symptoms, the patient can be followed clinically; generally there is no need for angiographic follow-up.
Without treatment, carotid—cavernous fistulas take an unfavorable course. Besides oculomotor impairment due to compression of cranial nerves III—VI in the cavernous sinus, the chemosis and venous reflux pose a threat to the eye. Eventually, moreover, the cavernous sinus and ophthalmic vein on the opposite side become pathways for fistulous drainage via the intercavenous sinus, posing a danger to the other eye as well. Stealing of blood by the fistula threatens the blood supply to the brain and can lead to ischemic cerebral dysfunction and damage. The arterialization of cortical veins leads to a (relatively low) risk of intracerebral or subarachnoid hemorrhage. For all these reasons, the endovascular therapy of a traumatic carotid—cavernous fistula should be instituted within a short time after the primary treatment of the trauma patient.
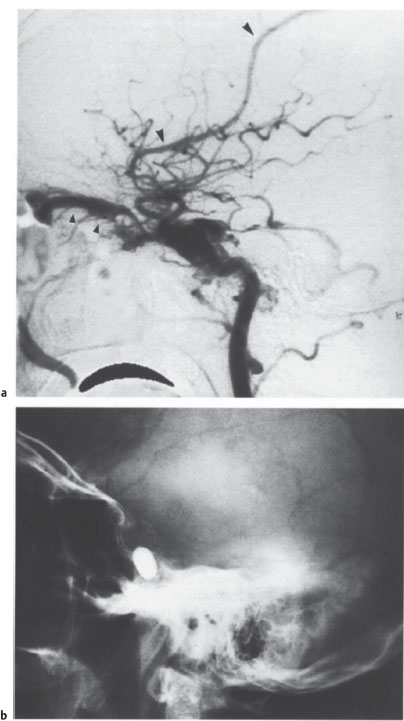
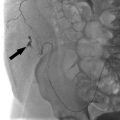
Fig. 4.2 a–c Endovascular occlusion of a traumatic carotid—cavernous fistula.
a on the lateral carotid angiogram, contrast injection produces immediate opacification of the cavernous sinus, superior ophthalmic vein (small arrowheads), and a cortical vein (large arrowheads), signifying an abnormal connection between the artery and venous system.
b Lateral skull radiograph after endovascular therapy shows the detached, contrast-filled balloon that was placed in the cavernous sinus to occlude the fistula.
c Lateral follow-up angiogram confirms total occlusion of the fistula with preservation of the carotid lumen. The slight residual stenosis of the internal carotid artery is not hemodynamically significant. The arrowheads indicate the balloon.
 Indirect Carotid—Cavernous Fistulas
Indirect Carotid—Cavernous Fistulas
Indirect carotid—cavernous fistulas arise spontaneously. Their symptoms are similar to those of direct fistulas but are less severe and often develop over a period of months. They may be fed by all the arteries that supply the dura along the cavernous sinus, i.e., the dural branches of the internal and external carotid arteries on both sides. Due to the small volume of the AV shunt and the slow flow, the feeding arteries and draining veins show very little dilatation. The feeders and also the (often numerous) AV connections are too small for occlusion with balloon catheters.

Particle embolization does not provide permanent occlusion and should be considered obsolete.
The only promising technique is superselective catheterization and embolization of the feeding arteries and fistulous connections with tissue adhesives such as Histoacryl and Ethibloc.
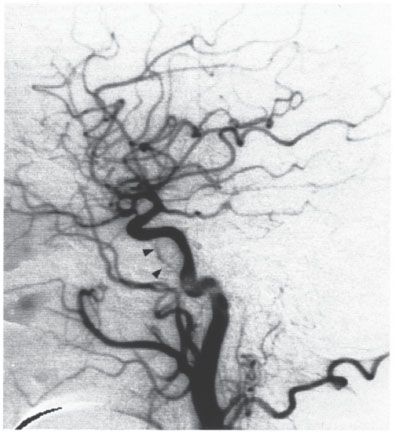
Vertebral Artery Fistulas
Vertebral artery fistulas may arise spontaneously, as a result of trauma, or as an accompanying feature of certain systemic diseases such as neurofibromatosis I and fibromuscular dysplasia (its pathogenesis in these diseases is uncertain). The lesions consist of solitary AV connections with varying calibers, flow velocities, and hemodynamic effects.
Spontaneous fistulas are probably congenital and are generally manifested in childhood by a bruit that is synchronous with the pulse. The large flow volume across the shunt can lead to vertebrobasilar insufficiency. Traumatic fistulas occur after stabbings or gunshot injuries or may follow percutaneous needle procedures in the neck. Typically the orifice of the fistula is large, resulting in a high shunt volume. The treatment of choice is occlusion of the fistula with a detachable balloon that preserves the lumen of the parent vessel (Fig. 4.3).
Dural AV Fistulas
Dural AV fistulas are acquired
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree