Fig. 22.1
(a) Introduction of delivery device (Schematic of Parodi JC stent—graft 1991). (b) Deployment of device (Schematic of Parodi JC stent—graft 1991)
1 Anatomy
Understanding aorto-iliac anatomy is important for the successful performance of EVAR.
Most AAA are infrarenal. However, up to 15% of AAA may have a juxtarenal or suprarenal component. Currently available FDA-approved devices can be used to treat most infrarenal AAA; however, fenestrated grafts or hybrid procedures are required to treat suprarenal AAA. In the USA fenestrated grafts are currently available only on a trial basis. Some of these devices are more readily available outside the USA.
A second anatomic factor of importance relates to the landing zone and the adequacy of the neck. A “neck” refers to the distance from the origin of the most distal renal artery and the beginning of the aneurysm. Currently most devices can treat AAA with a neck of up to 3.2 cm in diameter and 15 mm in length. The talent device is approved to treat shorter (10 mm) necks. The ideal neck is parallel, with no angulation or eccentric thrombus in the wall. 45–60° angulation is the maximum suggested by the device manufacturers (Fig. 22.2). In cases where the diameter is not uniform throughout the (conical) neck, up to a 3 mm difference in diameter is well tolerated. The presence of an accessory or duplicated renal artery may reduce the neck length. In these cases the possibility of covering an accessory renal artery and the possibility of partial renal ischemia has to be carefully considered. Other factors, which are important in terms of the adequacy of the neck, are tortuosity of the aorta and the presence and nature of thrombosis at the proximal seal zone. All devices today are bifurcated and distally the stent is landed on the iliac arteries. Distally adequate seal zones require adequate arterial length and diameter to prevent a distal type I endoleak. 20–30% of infrarenal AAA have common iliac artery aneurysms. If the common iliac arteries are short or aneurysmal, the devices may require external iliac artery deployment. In these cases the internal iliac artery or arteries may need to be embolized to prevent a type II endoleak; an internal iliac branched graft may be used in limited cases. Adequacy of collateral blood supply related to previous surgical history to the colon and pelvic organs has to be taken into account. A large patent IMA may be cause for a type II endoleak and in some centers this vessel may be embolized preoperatively.
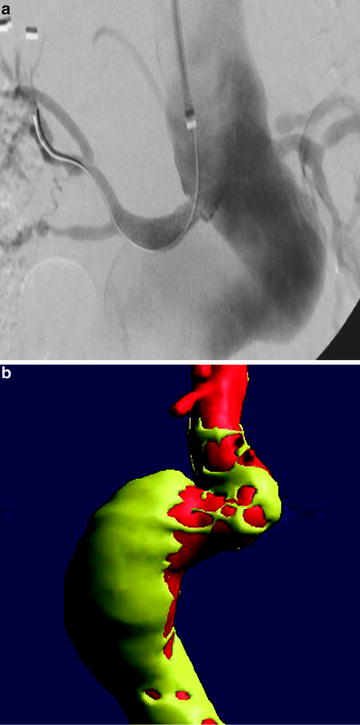
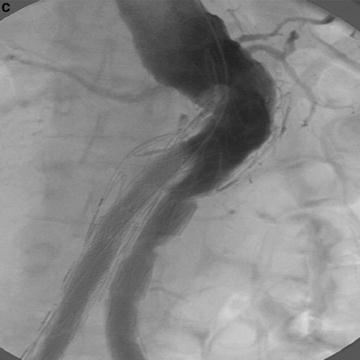


Fig. 22.2
(a) Treatment of AAA with talent device. Marked tortuosity of proximal aortic neck presented significant challenge to endovascular treatment. (b) Treatment of AAA with Talent device. CTA 3D reconstruction. (c) Treatment of AAA with Talent device. Use of proximal extension prosthesis permitted device to conform to aortic neck and prevent endoleak at proximal fixation site
The third anatomic factor of importance relates to the access vessels. Standard deployment of stent grafts requires placement of large diameter sheaths in the common femoral arteries. Vessels of at least 6 mm are required for the current available devices. In addition, tortuousity of femoral and iliac vessels may impede passage of the device. Although the use of stiff wire may allow some straightening of these vessels, care must be taken to prevent risk of arterial rupture. Severe calcification (circumferential) of the vessels may also impede advancement of the device. Techniques such the use of an iliac conduit, may allow for passage of the device. If one iliac vessel is simply not amenable, an aorto-uni device and fem–fem bypass may be considered.
2 Disease Definition
An aneurysm is defined as a 50% enlargement of a vessel. The normal infrarenal aorta measures 2 cm on average in men and 1.8 cm in women. The normal common iliac artery measures approx 1.2 cm in men. Most AAA are fusiform as opposed to saccular which are slightly more frequent in thoracic aortic aneurysms (TAA).
In regards to etiology, more than 90% of aneurysm is degenerative. Although the term “atherosclerotic” is still commonly applied to such aneurysms, atherosclerosis per se is not a direct cause. Complex processes are associated with the pathophysiology of these aneurysms. Other causes of AAA are inflammatory or mycotic in nature. Inflammatory aneurysms that are not infectious in etiology are potentially amenable to EVAR. Mycotic aneurysms are not treated with EVAR since the focus of infection is still present. The exception is an emergency. EVAR can be used as a temporary bridge to definitive repair for ruptured mycotic aneurysms. AAA is repaired to prevent rupture which carries a high mortality rate. Size is the major factor associated with aneurysm rupture. The relationship of AAA diameter and rupture risks is described subsequently. In addition to size, the aneurysm’s rate of increase over time, the patient’s cigarette usage, and life expectancy play a role in the decision to operate as opposed to the decision to observe. Clinical trials have been conducted comparing observation versus repair.
3 Disease Distribution
There are 27 million AAA patients worldwide. The prevalence of AAA in the over 50 population ranges from 3% to 10% in multiple screening and autopsy studies performed in the USA and internationally. In a VA-screening study of 73,000 patients the prevalence of AAA is 4.6% among patients from ages 50 to 79. The rupture of an AAA is the 15th leading cause of death in the USA and the 10th leading cause of death in men older than 55. These numbers have steadily increased since prevalence studies were performed starting 50 years ago. The most significant risk factors for AAA are smoking, age, gender, family history, and race. Men have a two to six times higher frequency of AAA than women. Caucasians have a two to higher times frequency of AAA than non-Caucasians. Other less important risk factors are hypertension and hypercholesterolemia.
The overall mortality of patients with ruptured AAA is 80–90%. 30–50% of patients with ruptured AAA die prior to reaching the hospital. 30–40% of patients die after reaching the hospital without any intervention. There are approximately 40,000 elective AAA repair in the USA every year.
Operative mortality rates for ruptured AAA range from 40% to 50% though there is increasing evidence that mortality with endovascular repair will be lower.
4 Diagnosis
Physical examination is very limited as a form of diagnosis in this condition. The positive predictive value of physical examination is only 15%. Most AAA are found either incidentally during the course of radiologic examinations for other reasons or, more recently, from screening programs. Multiple studies of ultrasound (U/S) screening have shown benefit. Historically, most of these studies come from the UK. Today in the USA, Medicare reimburses, a one time screening for males 65 years and older. Screenings with a second ultrasound have not proved to add benefit. Routine screenings for women are not generally recommended in the USA though screenings of women with specific risk factors, such as a positive family history or a history of tobacco use are beneficial. The most common confirmatory test for physical exam results is B-mode U/S. B-mode U/S is non-nvasive, inexpensive and therefore commonly used for follow-up of small AAA. When the AAA reaches a size where repair is considered, a thin cut (3 mm) CT angiogram (CTA) is the test of choice. If a repair is indicated, the CTA is useful in deciding upon whether the repair should be open or endovascular. MRA is slightly more expensive than CTA and requires more time for imaging, but it may be used in lieu of CTA in specific circumstance such as when platinum coils from a previous embolization limits the benefit of CTA, or if the patient has an iodine dye allergy. Angiography is usually performed when a specific preoperative intervention may be required, such as pre-EVAR embolization of a hypogastric artery or the definition of renal vascular anatomy in cases where the patient has more than one renal artery. Digital subtraction angiography is rarely required at present for diagnostic purposes and frequently underestimates the aneurysm diameter.
Alternative approaches such as CT without contrast, CO2 as contrast or intravascular US (IVUS) may be utilized for preoperative planning in patients with renal insufficiency. However the EVAR procedure itself requires contrast, therefore the renal function itself must be taken into account as comorbidity when evaluating a patient for surgery.
5 Management
Repair of an asymptomatic AAA is prophylactic and elective. The decision for repair must weigh the risk of AAA rupture on the one hand with the operative risk and the patient’s life expectancy on the other. A thorough discussion of each of these factors with the patient is necessary for the patient to give informed consent.
Although not ideal, the primary determinant of AAA rupture used in practice is aneurysm size. In the UK small aneurysm trial (UKSAT) annual rupture risk was found to be 0.3% (3.9 cm or smaller), 1.5% (4–4.9 cm), and 6.5% (5–5.9 cm). These finding apply mostly to men who encompassed 85% of the study population. Other studies with similar numbers document rupture rates of 10–20% (6–7 cm) and 20–40% (>7 cm). (Table 22.1) Advances in predicting the risk of rupture include models of aneurysm wall stress and finite element analysis.
Table 22.1
Range of potential rupture rates for a given size of abdominal aortic aneurysm
AAA Diameter (cm) | Rupture risk (%/year) |
|---|---|
<4 | 0 |
4–5 | 0.5–5 |
5–6 | 3–15 |
6–7 | 10–20 |
7–8 | 20–40 |
>8 | 30–50 |
Less significant factors influencing the risk of rupture include female gender and family history, smoking (active as well as past history) and the rate of the aneurysm’s expansion. Predicted rate of expansion is 10% of size per year. An expansion rate beyond 10% may assist in the recommendation for repair in smaller aneurysm.
Two major studies, The UK small aneurysm trial (UKSAT) and the aneurysm detection and management study (ADAM), have demonstrated a negligible risk of rupture in AAA less than 4 cm. These aneurysms may be followed with U/S. There was a clear benefit of AAA repair in aneurysms larger than 5.5 cm. Recommendation for follow up versus operative intervention in aneurysms 4–5.5 cm may vary according referral patterns, gender, and rate of expansion
In regards to endovascular repair, recently, two trials the CAESAR in Europe and the PIVOTAL study in the USA are in the process of studying the risk of rupture versus endovascular repair in aneurysms smaller than 5.5 cm. Operative mortality of endovascular repair is lower than for open repair and overall EVAR outcomes (in retrospective reviews) have been shown to be better in smaller AAA.
In terms of operative risk, open repair has had a steady 4% mortality rate over the last few decades. EVAR has shown an operative mortality rate of approximately 1% in multiple studies. For this reason, the decision to intervene will be different if the patient is not a candidate for endovascular repair. Given the low mortality rate for EVAR, prediction algorithms, which take into account the patient’s comorbidities, have not been found to contribute significantly to the calculation of operative risk.
Calculating the life expectancy for candidates of AAA repair is not simple. Factors such as the impact of the procedure itself, the patient’s comorbidities (both associated and independent from AAA), and age of the patient are all contributing factors. (Table 22.2)
Table 22.2
Life expectancy in years for patients surviving abdominal aortic Aneurysm repair by age, gender, and race
Age (yr)
Total
Male
Female
White
Black
White
Black
60
13
12
11
14
13
65
11
11
10
12
11
70
10
9
8
10
10
75
8
8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access