Approximately 20% to 40% of patients who have cerebral vascular disease have a vertebral artery–origin stenosis. Atherosclerotic lesions of vertebral arety origin are a potential cause of posterior circulation ischemia, which can be disabling or deadly. Endovascular treatment of vertebral artery–origin and innominate/subclavian artery stenosis has changed in the last 15 years. Surgery usually is successful technically; however, it is also associated with high rates of procedural and periprocedural complications. New techniques and technologies that can be used in the treatment of such lesions are being developed. In this article, the authors discuss the indications, technical aspects, and long-term results of angioplasty and stenting of these vessels.
Extracranial vertebral artery (VA) atherosclerotic disease most frequently involves the origin of the vessel. The prevalence of VA-origin stenosis varies from approximately 20% to 40% of patients who have cerebrovascular disease . Atherosclerotic lesions of VA origin are a potential cause of posterior circulation ischemia, which can be disabling, or even deadly. Of patients experiencing posterior circulation transient ischemic attack (TIA), 22% to 35% will suffer an infarct within 5 years of the TIA, with a mortality rate of 20% to 30% . Patients who have atherosclerotic disease of VA origin are treated initially with antiplatelet medications or anticoagulation. A high degree of stenosis (greater than 50%) or failure of medical therapy prompts intervention, either open surgery or endovascular procedures. Surgery usually is successful technically; however, it is also associated with high rates of procedural and periprocedural complications . For these reasons, new techniques and technologies that can be used in the treatment of such lesions are being developed. Since the 1990s, percutaneous angioplasty and stenting have gained increasing importance as the first treatment modality for this patient population. Angioplasty and stenting of the VA origin, although technically simple, can be challenging, depending on the anatomic features of the aortic arch, brachiocephalic trunk, and subclavian arteries. Despite possible technical challenges, multiple clinical series and case reports have been published in the medical literature showing the feasibility, safety, and efficacy of this procedure in the treatment of symptomatic atherosclerotic lesions of VA origin .
Anatomy
The left VA frequently is larger than the right VA. The anatomy of the extracranial VA varies little between individuals. Usually, the VA is the first and largest branch of the subclavian artery, originating from its superior-posterior wall . The left VA arising directly from the aortic arch between the left common carotid artery and the left subclavian artery, rather than from the subclavian artery, is the most common variation (2%–5%). Beyond its origin, the VA runs behind the anterior scalenus muscle with a cephalad, slightly posterior trajectory, entering the foramina transversarium of C6 in 88% of people, C5 or C7 in 6% of people, and C4 in 4% of people .
The VA is divided into four segments. The first segment (V1) extends from its origin to its entrance into the foramina transversaria. The second segment (V2) extends from its entrance into the foramina transversaria to C2, and ascends almost straight, passing through the foramina transversarium of each vertebra to C2. As patients age, this segment can become tortuous and can be impinged on by bony spurs arising from the cervical spine. The third segment (V3) extends from C2 to the point at which the VA pierces the dura mater. Within this segment, the VA leaves the foramina transversarium of C2, forming a lateral convex curve, reaching the foramen of C1, and then running backward and subsequently antero-superiorly. The fourth segment (V4), also known as the intradural segment, extends from the point at which the VA enters the dura mater to the vertebrobasilar junction . Muscular branches originate from the second and third segments of the VA, supplying the cervical muscles with rich anastomoses to the thyrocervical and costocervical trunks and the external carotid artery . The knowledge of these anastomoses is extremely important because these anastomoses can provide an important collateral source for reconstitution of the distal VA in instances of severe proximal atherosclerotic disease.
Description and discussion of the rare anatomic variations of the subclavian and VA is beyond the scope of this article. The reader, however, should learn the possible locations of the subclavian and VA origins from imaging of the patient, including arch aortography.
Mechanism and clinical presentation
Vertebrobasilar insufficiency is an underdiagnosed clinical condition and usually is associated with nonspecific symptoms (such as dizziness) that also may be caused by other arterial territories .
Different mechanisms can cause symptoms of vertebrobasilar ischemia in the setting of extracranial VA stenosis, including artery-to-artery embolism and hemodynamic impairment when associated with stenosis, occlusion, hypoplasia, or absence of the contralateral VA . Another factor that needs to be considered is the characteristic of the VA-origin plaque. VA-origin plaque tends to be hard, smooth, concentric, and less prone to ulceration or intramural hemorrhage and, therefore, carries less risk of embolism, compared with carotid bifurcation plaque . Nevertheless, the proximal extracranial VA disease in the New England Medical Center Posterior Circulation Registry, where data from 407 consecutive patients were evaluated, revealed that 20% of these patients had lesions in the V1 segment of the VA. The analysis of these data demonstrated that the cause of posterior circulation ischemic events was artery-to-artery embolism from VA origin in 24% of the population studied, and that this number increased to almost 50% when possible artery-to-artery embolism cases were added . The same study also showed that hemodynamic TIA due to bilateral vertebral disease was a less common, but significant, mechanism, accounting for 16% of the study population .
Embolism usually affects the distal circulation with a sudden onset of maximal neurologic symptoms and signs, which may resolve spontaneously (TIA). An infarct with an embolic source is more likely to result in parenchymal hemorrhage than an infarct with a hemodynamic cause . Some investigators state that hemodynamic impairment is the most important mechanism of infarct formation when embolic cardiac sources are excluded . Hemodynamic symptoms usually result from stenosis when associated with occlusion, hypoplasia, absence, or severe stenosis of the contralateral VA, limiting the blood flow to the parenchyma in the vertebrobasilar distribution. The resulting decrease in perfusion and the degree of collateral circulation determine the occurrence of symptoms. Even asymptomatic patients can become symptomatic if the arterial blood pressure is decreased, thereby decreasing the parenchymal perfusion .
Multiple symptoms have been associated with atherosclerotic disease of VA origin resulting in vertebrobasilar ischemia, including posterior circulation strokes and persistent symptoms of vertebrobasilar insufficiency. Dizziness, vertigo, impaired balance, dysarthria, syncope, diplopia, ataxia, nausea, vomiting, tinnitus, cortical blindness and visual deficit, memory disturbance, nystagmus, motor or sensory complaints, facial palsy and numbness, dysphagia, mental status changes, and decreased level of consciousness are all symptoms and signs that may be related to vertebrobasilar ischemia .
Mechanism and clinical presentation
Vertebrobasilar insufficiency is an underdiagnosed clinical condition and usually is associated with nonspecific symptoms (such as dizziness) that also may be caused by other arterial territories .
Different mechanisms can cause symptoms of vertebrobasilar ischemia in the setting of extracranial VA stenosis, including artery-to-artery embolism and hemodynamic impairment when associated with stenosis, occlusion, hypoplasia, or absence of the contralateral VA . Another factor that needs to be considered is the characteristic of the VA-origin plaque. VA-origin plaque tends to be hard, smooth, concentric, and less prone to ulceration or intramural hemorrhage and, therefore, carries less risk of embolism, compared with carotid bifurcation plaque . Nevertheless, the proximal extracranial VA disease in the New England Medical Center Posterior Circulation Registry, where data from 407 consecutive patients were evaluated, revealed that 20% of these patients had lesions in the V1 segment of the VA. The analysis of these data demonstrated that the cause of posterior circulation ischemic events was artery-to-artery embolism from VA origin in 24% of the population studied, and that this number increased to almost 50% when possible artery-to-artery embolism cases were added . The same study also showed that hemodynamic TIA due to bilateral vertebral disease was a less common, but significant, mechanism, accounting for 16% of the study population .
Embolism usually affects the distal circulation with a sudden onset of maximal neurologic symptoms and signs, which may resolve spontaneously (TIA). An infarct with an embolic source is more likely to result in parenchymal hemorrhage than an infarct with a hemodynamic cause . Some investigators state that hemodynamic impairment is the most important mechanism of infarct formation when embolic cardiac sources are excluded . Hemodynamic symptoms usually result from stenosis when associated with occlusion, hypoplasia, absence, or severe stenosis of the contralateral VA, limiting the blood flow to the parenchyma in the vertebrobasilar distribution. The resulting decrease in perfusion and the degree of collateral circulation determine the occurrence of symptoms. Even asymptomatic patients can become symptomatic if the arterial blood pressure is decreased, thereby decreasing the parenchymal perfusion .
Multiple symptoms have been associated with atherosclerotic disease of VA origin resulting in vertebrobasilar ischemia, including posterior circulation strokes and persistent symptoms of vertebrobasilar insufficiency. Dizziness, vertigo, impaired balance, dysarthria, syncope, diplopia, ataxia, nausea, vomiting, tinnitus, cortical blindness and visual deficit, memory disturbance, nystagmus, motor or sensory complaints, facial palsy and numbness, dysphagia, mental status changes, and decreased level of consciousness are all symptoms and signs that may be related to vertebrobasilar ischemia .
Diagnostic work-up
The VA origin is a segment difficult to image . Color Doppler ultrasound may offer good views, providing important diagnostic and hemodynamic information . Developments in the protocols of imaging modalities such as CT angiography and MR angiography are improving the visualization of the VA origin, allowing faster diagnosis of VA-origin disease. Catheter angiography remains the gold standard for quantification of the degree of stenosis at the VA origin, evaluation of the plaque, detection of ulceration or thrombus, and assessment of the extra- and intracranial collateral circulation. A complete four-vessel study is essential to evaluate possible lesions in the carotid arteries, subclavian arteries, and intracranial vessels; it also identifies VA dominance and gives important clues regarding the mechanism of vertebrobasilar ischemia (embolic versus hemodynamic).
A cross-sectional study of the brain parenchyma with CT, or preferably MR imaging, should be performed to assess for acute or old infarcts, ischemia, or hemorrhage, because these findings will have a major impact in the decision-making process.
Indications
Classically, vertebrobasilar insufficiency is treated initially with antiplatelet agents, anticoagulants, or a combination of both (see Refs. ). Medical treatment regimens for vertebrobasilar insufficiency are derived from carotid studies’ data; unfortunately, it is not clear if medical therapy has any benefit or if it should be the first line of treatment . When optimal medical therapy fails to prevent posterior circulation ischemic symptoms, endovascular techniques can be proposed. The reason is that, in these selected cases, the potential benefits of the endovascular treatment (angioplasty and stenting) outweigh the risks of the procedure.
At our institution, patients presenting with posterior circulation ischemic symptoms despite optimal medical therapy, and who have a digital subtraction angiogram demonstrating VA-origin stenosis greater than 50%, are considered for endovascular therapy.
If the source of posterior circulation ischemic events is thought to be embolic and no other sources of arterial embolism are found (cardiac), it is reasonable to assume that the symptoms are the result of artery-to-artery embolism from the diseased VA origin. For this reason, even if the stenosis is less then 50%, we believe that it should be considered for treatment because it may be the source of embolism. The neointima that develops following angioplasty and stenting may protect against future distal embolism by smoothing luminal irregularities, thereby decreasing flow turbulence at the origin of the vessel .
The treatment of asymptomatic patients who have significant stenosis of VA origin is a subject of controversy. Although most asymptomatic patients do not require endovascular treatment, some investigators believe that high-grade stenosis (greater then 70%) affecting the origin of a dominant or single VA should be treated because of a possible increased risk of stroke . Other investigators believe asymptomatic patients should be treated when the necessity of collateral support is of major importance (as in cases of carotid occlusion) . VA stenosis may become symptomatic when hypertensive patients, or even normotensive patients, have a reduction in their blood pressure.
Technical aspects
All patients should have a complete history elicited and a neurologic examination for intra- and postprocedure comparison. A complete cervicocranial angiogram should be performed to evaluate the target lesion, the collateral pathways, the direction of flow, and the presence of asymptomatic stenosis that could influence the decision process for the procedure.
As per our protocol, all elective patients are pretreated for 5 days with clopidogrel bisulfate 75 mg per day, in addition to aspirin, 325 mg per day. The antiplatelet pretreatment regimen is extremely important to decrease the risk of stent thrombosis after endothelial injury, or plaque rupture following angioplasty and stenting . In urgent cases, patients can be loaded with 300 mg of clopidogrel bisulfate in addition to aspirin on the day of the procedure. Another option is a bolus infusion of glycoprotein IIb/IIIa inhibitors concomitantly with a loading dose of oral antiplatelet agents just before the procedure.
An interesting procedural point is the use of conscious sedation versus general anesthesia. Most of our procedures are performed using local anesthesia and conscious sedation, which allows serial neurologic examinations throughout the procedure, enabling recognition of procedural complications and hastening their treatment. We reserve general anesthesia for patients for whom problems with airway control or patient motion are anticipated.
The arterial endovascular access is usually transfemoral (most common) or transbrachial (in cases of unfavorable VA-origin angle for the transfemoral approach). Recently, the transradial approach has been proposed . Its advantages include easy hemostasis and comfort to the patient (the patient is able to sit and walk immediately after the procedure) . The patient must have adequate ulnar arterial supply to the hand, so that occlusion of the radial artery will not result in an ischemic hand. Ulnar arterial supply is assessed before the procedure with the Allen test and possibly Doppler sonography.
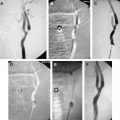
After arterial access has been obtained and an appropriately sized arterial sheath secured in place, systemic heparinization is begun. According to our anticoagulation protocol, all patients are heparinized during the procedure with intravenous heparin, a 70–100 U/kg bolus followed by a 7–10 U/kg per hour infusion, with a goal of an activated clotting time of 250 to 300 seconds. Using a standard hydrophilic guide wire and a 6F guide catheter, the target subclavian artery is catheterized and the guide catheter advanced to just proximal to the VA ( Fig. 1 ). The 6F guide catheter usually provides adequate stability. In cases of marked tortuosity of the subclavian artery or brachiocephalic trunk, an 80-cm 6F shuttle sheath may be necessary to provide a stable platform. If the brachiocephalic or subclavian arteries cannot be catheterized with the curved guide catheter, or if the shuttle sheath is necessary, the target brachiocephalic or subclavian artery is selected with a diagnostic catheter. Then, with the use of a 0.035-in exchange-length guide wire, the diagnostic catheter is removed and replaced with the guide catheter or shuttle sheath. This procedure requires some forethought because the groin or brachial sheath needs to be sized to allow placement of the guide catheter, or removed for placement of the long shuttle sheath. For further stability and better control, a 0.014-in or 0.018-in guide wire may be placed in the ipsilateral axillary artery through the side port to support the guide catheter and prevent it from moving ( Fig. 2 ). After the guide catheter or sheath is stable in the subclavian artery, an angiogram is performed for quantification of the stenosis and measurement of the VA. The best view of the stenosis is then selected as a working projection. The degree of stenosis is determined in relation to the diameter of the normal segment of vessel immediately distal to the stenosis. Biplane road map images are then obtained and the stenosis is crossed with a curved-tip 0.014-in or 0.018-in guide wire. The curved tip helps to negotiate the stenosis and prevent subintimal dissection at the site of stenosis or distally within the VA. The wire tip is positioned in the distal cervical VA within the fluoroscopic field-of-view, providing additional stability to the system.