Clinical Presentation
The patient is a 75-year-old female with complaints of generalized pain and pleural effusions. The patient began experiencing dyspnea on exertion 5 months ago and was discovered to have a large pleural effusion. This was drained and then a month later a recurrent effusion was drained. Over the next few months she began to experience left-sided pain in the region of the chest tube and subxiphoid pain. Over the past 2 weeks, the patient has noticed a mildly tender left-sided neck mass. Since last night, the patient has been noticing a sensation of pins and needles in her legs while trying to stand up, with significant leg weakness and imbalance.
Imaging Presentation
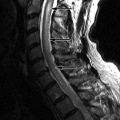
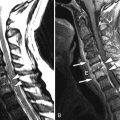
Sagittal and axial fat-saturated, post-contrast, T1-weighted images reveal abnormally enhancing soft tissue in the epidural space that is compressing the thecal sac. Two vertebrae reveal pathologic enhancement. The findings are compatible with metastatic disease to bone and epidural neoplasm ( Fig. 31-1 ) .

Discussion
Spinal cord compression from spinal epidural metastasis (SCCSEM) is a common complication of malignancy affecting approximately 5% of cancer patients. SCCSEM is a medical emergency that if left untreated will invariably lead to paraplegia. However, with early diagnosis and treatment, the effects of spinal cord compression can be prevented or reversed in most patients. SCCSEM is second only to brain metastasis as the cause of neurologic dysfunction caused by metastasis.
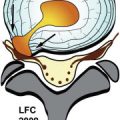
Metastatic spinal disease can arise from three different locations: the vertebral body (85%), the paravertebral region (10%-15%), and although rarely, the epidural space itself (less than 5%). Prostate, breast, and lung cancers each account for 15% to 20% of all spinal metastases and SCCSEM cases. Non-Hodgkins is lymphoma, renal cell carcinoma, and multiple myeloma each account for 5% to 10% of cases, and the remainder are primarily due to colorectal cancers, thyroid cancer, and sarcomas. The location of SCCSEM appears to be related to the relative bone mass and blood flow to each segment of the spine, with 60% of SCCSEM occurring in the thoracic spine, 25% in the lumbar spine, and 15% in the cervical spine. Spinal epidural metastases are reported to be multiple in 17% to 30% of patients.
Most cases of SCCSEM (85%) occur through entrance into the epidural space from a vertebral metastasis. Historically, it was thought that metastatic disease to the vertebral body occurred through the Batson’s plexus, the valveless venous system of the spine. However, there has been more recent work that suggests that arterial embolization is the most common and important route of vertebral metastasis.
SCCSEM can damage the spinal cord through, most importantly, vascular compromise and secondarily by direct compression. Anterior spinal cord compression can result in occlusion of Batson’s plexus resulting in vasogenic edema. If compression continues, and arterial flow to the spinal cord becomes impaired, the cord may undergo ischemic change and eventually infarction.
Pain is the most common manifesting symptom with SCCSEM, occurring in approximately 90% of patients. Local spine pain is confined to the region of metastatic disease and is thought to be due to periosteal stretching and/or a local neoplastic inflammatory process. Local pain is often made worse by recumbency caused by the lengthening of the spine and distention of the epidural venous plexus. Radicular pain is often made worse with Valsalva maneuvers. Mechanical back pain is often secondary to a vertebral body compression. These fractures tend to be associated with spinal instability, made worse with movement, and relieved with recumbency.
Weakness is the second most common symptom of SCCSEM, seen in 60% to 85% of patients at the time of diagnosis. Two-thirds of patients with SCCSEM are nonambulatory at the time of diagnosis. Bowel and bladder symptoms are seen in 50% to 60% of patients with SCCSEM at the time of diagnosis and parallel the degree of generalized weakness. Sphincter problems are a poor prognostic sign for the preservation or improvement of ambulation.
The diagnosis of SCCSEM begins with clinical suspicion. Any new-onset back or neck pain in a patient with a known history of cancer, especially of the prostate, breast, or lung, should prompt an evaluation for SCCSEM. This is even more concerning in a patient with thoracic back pain because the thoracic spine is much less commonly involved by degenerative back pain such as in the cervical and lumbar spine. A study by Kienstra and colleagues indicated that identifying patients at risk for SCCSEM was not possible with the standard neurologic checkup and therefore imaging, particularly MRI of the entire spine should be performed.
Imaging Features
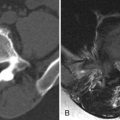
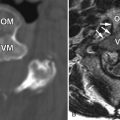
MRI is the imaging method of choice for evaluating metastatic disease and SCCSEM because it is nonionizing and can survey the entire spine quickly. Computed tomography (CT), particularly CT post-myelography, is still used in patients with contraindications to MR examination. Sagittal T1-weighted and sagittal fat-saturation or water excitation T2-weighted acquisitions of the cervicothoracic and thoracolumbar spine can be initially performed. Axial acquisitions and potentially contrast-enhanced acquisitions can then be performed at localized areas of abnormality. Typically, SCCSEM appears as a region of decreased T1 signal, increased T2 signal, and enhancement ( Fig. 31-2 ) . MRI is able to depict the relationship of the tumor to the spinal cord and neural foramen and whether or not there is abnormal increased T2 signal intensity in the spinal cord to suggest compressive edema, ischemia, or infarction ( Fig. 31-3 ) . MRI also adds information that is often not detected by CT, and for 40% of patients, the MRI information changes the radiation fields being used in treatment. CT can detect vertebral body destruction by metastatic disease and associated soft tissue masses as well as intraspinal and paraspinal metastases, although not as well as MRI ( Fig. 31-4 ) . The addition of intrathecal contrast during myelography with a follow-up CT scan has a higher sensitivity for intraspinal abnormalities. Vertebral metastases and epidural metastases are of low to intermediate density. Nuclear medicine positron emission tomography (PET) imaging can detect regions of metastatic disease, and when fused with CT data, can localize the abnormality. A bone scan is able to detect only bone metastasis and not the adjacent soft tissue abnormalities. Plain film radiographs should not be used as a primary diagnostic test for SCCSEM because they are falsely negative in 10% to 17% of cases and greater than 50% of the bone must be destroyed before an abnormality can be detected.