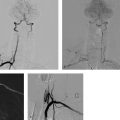
10 Equipment: Stents, Filters, and The techniques for the treatment of intracranial and extracranial stenoses are fundamentally different. The equipment for these techniques is therefore discussed under separate headings. Technical standards like those used in cerebral angiography also apply in the endovascular treatment of cerebrovascular stenoses (continuous irrigation of the sheath and catheter by pressure infusion, anticoagulation, etc.; Thiex et al. 2010). Elective procedures also require appropriate and timely preparations, including a stock check to make sure that the necessary equipment is available with minimal redundancy, because occasionally items may be inadvertently damaged or contaminated during preparations. Repeating a treatment also poses a repeated risk to the patient—a risk that is not justified by avoidable errors such as the use of faulty or incorrect supplies. Practical Tip Checklist (review on previous day, at the latest): • Long (70–90 cm) reinforced sheath (6–8F) • Coaxial catheter (6–8F) • Guidewire (e.g., Terumo 0.035 in [0.89 mm]) • Microwire • Stent (diameter, length, type—inventory) • Dilatation balloon (length, diameter) • Pressure syringe for dilatation • Premedication: anticoagulant and antiplatelet drugs Companies that develop and market these products are listed in Table 10.1. A long sheath (70–90 cm), flexible but adequately stiff in its proximal and middle segments, is recommended. This is necessary to ensure a stable position in the aortic arch. A sheath used for treatments in the carotid artery and its branches should extend into the common carotid artery, while a sheath used for treatments of the vertebral and basilar arteries should extend into the subclavian artery. For enhanced stability, sheaths are available that have a braided shaft or are reinforced with coiled wire. The sheath is placed atraumatically at the supraaortic level by advancing it from the aortic arch into the target vessel over a coaxial catheter with a hydrophilic-coated standard guidewire (diameter usually 0.035 in [0.89 mm]). The hydrophilic coating on the wire serves to reduce the thrombogenicity of the device and reduce friction within the catheter and along the vessel wall during insertion. To achieve these properties, the wire must be adequately hydrated before use. Caution A hydrophiliccoated wire will also slide more easily into smaller arteries than a standard wire. Thus, advancing the coated wire against resistance carries an increased risk of vascular dissection or perforation. A microwire, usually with a hydrophilic coating, is most often used for the navigation of stents and dilatation balloons. The wire must have good flexibility and should be stable over its entire length. Only the last few centimeters before the atraumatic tip are very soft (diameter 0.014–0.021 in [0.36–0.53 mm], depending on the stent system used). Self-expanding stents are most commonly used for the treatment of extracranial stenoses in the internal carotid artery, common carotid artery, and the V2 or V3 segment of the vertebral artery. Balloon-expandable stents are used only for rare indications—mainly stenoses affecting the brachiocephalic trunk, the left common carotid artery at its origin from the aortic arch, or the V0 or V1 segment of the vertebral artery. After a self-expanding stent has been placed, a balloon is generally used to dilate the stenosis and appose the stent to the vessel wall. Manufacturers offer various protection systems that are designed to shield the brain from treatment-associated thromboembolism. To date, however, studies have not demonstrated a benefit from the use of embolic protection systems (Barbato et al. 2008), even though they are increasingly used throughout the world, especially in the treatment of internal carotid artery stenoses. Table 10.1 Manufacturers of endovascular materials (listed alphabetically)
Balloon Systems
Introduction
Materials for the Treatment of Extracranial Stenoses
Manufacturer | Web site | Products |
Abbott | Arterial occlusion system | |
Acandis | Intracranial stents | |
ARROW | Sheaths | |
BALT (Extrusion) | Coaxial catheters, microwires, microcatheters, intracranial stents | |
BARD | Stents | |
Boston Scientific | Stents, catheters | |
Boston Scientific Neurovascular | http://www.stryker.com/en-us/products/Neurovascularintervention/index.htm | Intracranial stents, microcatheters, microwires, dilatation catheters |
Codman & Shurtleff, Inc., | http://www.depuy.com/about-depuy/depuy-divisions/codman-and-shurtleff |
|
Concentric Medical | Guide catheters | |
Cook medical | Sheaths | |
Cordis | Guide catheters, stents, dilatation catheters | |
Covidien |
| |
Covidien/ev3 | Microcatheters, microwire, intracranial stents | |
Invatec | Stents, dilatation catheters, proximal protection system | |
Krauth Cardiovascular | Medical products distributor in Europe | |
Medtronic |
| |
Microvention | Coaxial catheters, microcatheters, microwires | |
Micrus | Coaxial catheters, microcatheters, microwires | |
OptiMed | Sheaths, stents | |
Penumbra | Coaxial catheters | |
St. Jude Medical | Arterial occlusion system | |
Stryker |
| |
Terumo | Guidewires |
Note: Although not complete, this listing includes most firms that manufacture or distribute products relating to the endovascular treatment of cervical and intracranial arteries supplying the brain. The internet addresses were up-to-date when this book went to press but, like the products themselves, are subject to change over time.
The three main groups of materials—stents, protection systems, and dilatation catheters—are described below with reference to carotid artery stenting.
Stents
Self-Expanding Stents
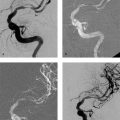
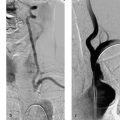
Both self-expanding and balloon-expandable stent systems are in use. Supra-aortic stenoses of the internal and common carotid arteries are most commonly treated with self-expanding stents—small meshwork tubes made from braided or woven monofilament wires (classic Wallstent; Fig. 10.1) or cut from a metal tube with a laser (Fig. 10.2.) Besides the traditional cobalt-chromium or cobalt-nickel steel, nickel-titanium alloy stents are also widely used. Nickel-titanium alloy (nitinol) has memory properties that increase the radial force of stents made of this material by converting thermal energy (body temperature) to mechanical energy.
Practical Tip
Stents cause variable susceptibility artifacts on MRI, depending on the type of alloy from which the stent is made. This may make it difficult or impossible to evaluate stented vascular segments on MRI follow-up, depending on the type of stent used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree