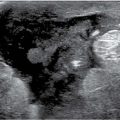
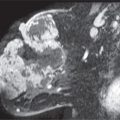
LEARNING OBJECTIVES
1. Patient history to consider when evaluating and considering differentials for a breast mass
2. Evaluation (imaging) algorithm for clinically or mammographically detected breast masses
3. Features of masses to consider and appropriate use of descriptors
4. Differential considerations for round (expansile) masses
5. Differential consideration for masses with spiculated, margins
6. Differential considerations for multiple masses in the breast with similar features
7. Differential considerations for fat containing masses
We place much emphasis on detecting microcalcifications and anguish over their characterization and management, but it is important to recognize that most invasive ductal carcinomas, and virtually all invasive lobular carcinomas, present as a mass. In contrast, when associated with malignancy, microcalcifications usually reflect intraductal, noninvasive breast cancer. Our primary goal with screening mammography is the recognition or perception of a possible mass or distortion related to an underlying mass. Our characterization of masses and management recommendations for patients are predicated on physical examination, spot compression, spot rolled, spot tangential, or spot compression magnification views and breast ultrasound. In some women, what appears to be a mass on screening images is shown to be superimposition of normal glandular tissue and what appears as an innocuous asymmetry is identified as likely malignant with further workup. By integrating patient history, clinical, mammographic, and ultrasound findings, and an understanding of breast histopathology, it is possible to approximate a diagnosis and have significant confidence in the appropriate management recommendations.
Relevant history is reviewed in evaluating and managing women with a breast mass. Women with a personal history of breast cancer or a history of breast cancer involving a first-degree relative (mother, father, sister, brother, daughter, or son) are at increased risk for developing breast cancer. This risk is increased if the relative developed breast cancer premenopausally or had bilateral breast cancer. The age and menopausal status of the patient, use of hormone replacement therapy, and physical findings should be known and factored in the formulation of a differential (Table 7.1).
Table 7.1 BREAST MASSES: RELEVANT HISTORY
Personal history of breast (or other malignancies, e.g., ovarian cancer) Family history of breast cancer First-degree relatives Premenopausal Bilateral Patient age (incidence of breast cancer increases with advancing age, and differential considerations change depending on age) Menopausal status (tumor sojourn time is shorter in premenopausal women) Hormone replacement therapy Tamoxifen use Physical findings “Lump” Focal pain Skin dimpling Nipple retraction Spontaneous nipple discharge History of cyclical change in primary finding History of surgical procedures involving the breast Prior breast biopsy with diagnosis of high-risk marker lesion ADH Lobular neoplasia (LCIS) |
In women over the age of 30 presenting with a focal finding (e.g., “lump,” dimpling, focal tenderness), a metallic BB is used to mark the area of concern, and mediolateral oblique (MLO) and craniocaudal (CC) views are obtained bilaterally (1). A spot tangential view of the focal finding is also obtained. Correlative physical examination and ultrasound are usually done. Ultrasound is not absolutely indicated if fatty tissue is imaged corresponding to the site of concern to the patient, and there is no chance that the area of concern has been excluded from the field of view (e.g., if the metallic BB is at the edge of the film, correlative physical examination and ultrasound are always done; see Figs. 3.1 and 3.2).
Physical examination and ultrasound are done in women who are pregnant, lactating, or under 30 years of age and present with a “lump” (see Figs. 4.45 and 4.47). If any concerns persist following this initial evaluation, an MLO may be done to exclude calcifications associated with an intraductal carcinoma that may not be apparent on ultrasound. If an underlying malignancy is suspected, mammographic images are obtained bilaterally to fully evaluate the patient.
As defined by the American College of Radiology Breast Imaging and Reporting Data System (ACR BI-RADS®), a mass is a “space-occupying lesion seen in two different projections.” This is to be distinguished from asymmetry, a term used for “an area of fibroglandular-density tissue that is visible on only one mammographic projection, frequently representing superimposition of normal breast structures” (2). Masses are three-dimensional, have a bulging or convex contour and an abrupt density change at the margin. They may produce architectural distortion and have associated calcifications, skin or nipple changes. Depending on location, size, internal matrix, and surrounding tissue, the mass may be palpable. Asymmetric tissue is planar with a different appearance between projections, scalloped and inhomogeneous with a gradual change in density at the margins. It is usually not palpable (3); palpable asymmetry should be evaluated carefully (see Chapter 9).
Compression, rolled and magnification views done with the round spot compression paddle (see Fig. 3.4A, B) are used to establish the shape, margins, and density of breast masses. Round, oval, and irregular (if “shape cannot be characterized”) are the terms in BI-RADS® to describe the shape of a mass. Circumscribed, microlobulated, obscured (margins “hidden by superimposed or adjacent normal tissue”), indistinct, and spiculated are the terms used to describe the margins of a mass. The x-ray attenuation or density of a mass is described as high, equal, or low (but not fat-containing) relative to an equal volume of fibroglandular tissue (Table 7.2) (2). Please see Appendix A for the ACR BI-RADS® mammography, US and MRI lexicons. Every rule has an exception; but in general, benign masses have circumscribed or partially circumscribed margins and are low to equal in density. In contrast, malignant lesions have indistinct or spiculated margins, and the expansile (round, oval, lobulated) masses are usually high in density.
Table 7.2 AMERICAN COLLEGE OF RADIOLOGY, LEXICON DESCRIPTIVE TERMS FOR MASSES
Shape | Margins | Density (Water Density) |
Round | Circumscribed | High |
Oval | Microlobulated | Low (but not fat-containing) |
Irregular | Obscured | Equal |
Indistinct | Fat-containing | |
Spiculated | ||
Sickles EA, D’Orse CJ, Bassett LW, et al. ACR-BIRADS® | ||
Table 7.3 MASSES: MAMMOGRAPHIC FEATURES TO CONSIDER
Shape Margins Density (low, equal or high density; no fat) Features of associated calcifications Effects on surrounding tissue Architectural distortion “Halo” sign Satellite lesions Multiplicity Stability (previous films) |
Additional features to consider when evaluating masses are listed in Table 7.3. Establishing the presence of associated calcifications and their morphology is helpful in assessing the etiology of a mass. Benign calcifications are usually associated with benign masses (Fig. 7.1). If malignant-appearing calcifications are associated with a mass, invasive ductal carcinoma with an intraductal component is the most likely diagnosis (Figs. 7.2 and 7.3). Likewise, establishing the presence of satellite lesions is helpful (Fig. 7.3). Although maligned, the halo sign is a good indicator of benignity (4–6). The halo sign is narrowly defined as a 1-mm sharp lucency, partially or completely surrounding a mass (Fig. 7.4). Not all masses with a true halo are benign but many are (4,5). The halo sign is probably as good a sign of benignity as spiculation is of malignancy. The halo sign may reflect active changes in the size of the mass (4).

FIG. 7.1 • Fibroadenoma, hyalinizing. Photographically coned image. An isodense oval mass (arrows) with indistinct margins and coarse, dense, dystrophic calcifications is imaged on the screening views (only one projection is shown). Although the margins are indistinct, the presence of dystrophic-type calcifications associated with the mass in both projections is consistent with a fibroadenoma undergoing hyalinization and calcification. Masses associated with benign calcifications are usually benign. When benign findings are detected on screening mammography, no additional evaluation is indicated. BI-RADS 2: Benign finding.
FIG. 7.2 • Invasive ductal carcinoma, not otherwise specified (NOS), with DCIS. A: A dense oval mass with indistinct margins and associated malignant-type calcifications is imaged (only one projection is shown). The calcifications are pleomorphic and include linear, round, and punctate forms. Given the density and margins of the mass (expansile, “blow-up” lesion), an invasive ductal carcinoma, high nuclear grade is likely, and the calcifications are consistent with associated DCIS with central necrosis. BI-RADS 5: Highly suggestive of malignancy, biopsy is indicated. B: Dense, irregular mass (long arrow) with indistinct margins and fine linear calcifications (short arrows) extending linearly from the posterior edge of the mass for approximately 3 cm. The mass reflects an invasive ductal carcinoma; the calcifications are consistent with DCIS with central necrosis. At the time of surgery, it is important to excise the mass and all of the calcifications; bracketing this entire area with at least two wires would be appropriate preoperatively. BI-RADS 5: Highly suggestive of malignancy, biopsy is indicated. Palpable or mammographically detected masses with linear-type calcifications most commonly reflect an invasive ductal carcinoma, NOS with associated DCIS.
The presence of multiple masses with similar mammographic features (Fig. 7.5) is suggestive of benignity. However, do not be lulled into a false sense of security by multiplicity. Women with multiple masses can develop breast cancer. It has been reported that since the frequency of cancer development among women not recalled for evaluation of multiple masses and the stage of the cancers diagnosed in these women is no different than that seen in the general screening population, evaluation of multiple masses appears not to be justified (7). Others advocate ultrasound evaluation in these patients (8). Although controversial, our approach to the patient presenting for the first time with multiple masses is to evaluate each mass as though it were a single finding. Our decisions are based on physical examination and the mammographic and ultrasound features of the masses evaluated. On subsequent mammograms in these patients we only evaluate new, developing masses. Also, don’t let yourself be mesmerized with multiple benign findings, but actively focus your brain away from the obviously benign findings and evaluate the surrounding tissue. Differential considerations for patients presenting with multiple masses is provided in Table 7.4.

FIG. 7.3 • Multifocal, invasive ductal carcinoma, not otherwise specified with DCIS. Dense, round mass with indistinct margins and fine, pleomorphic calcifications (short arrows) extending away from the mass at multiple sites such that invasive ductal carcinoma, high nuclear grade with associated DCIS with central necrosis is the likely diagnosis. A lower density round mass (long arrow) is present at the posterior edge of the dominant mass consistent with multifocal disease. BI-RADS 5: Highly suggestive of malignancy. A calcified artery (short thick arrows) is incidentally noted in the subcutaneous tissue.

FIG. 7.4 • “Halo sign,” cyst. A 1-mm sharp lucency (arrows) partially outlines this low density mass (arrows) with circumscribed margins. Although the halo sign can be seen with malignant masses that are growing rapidly, it is more common with benign masses. A cyst is imaged at this site on ultrasound (not shown). BI-RADS 2: Benign finding.
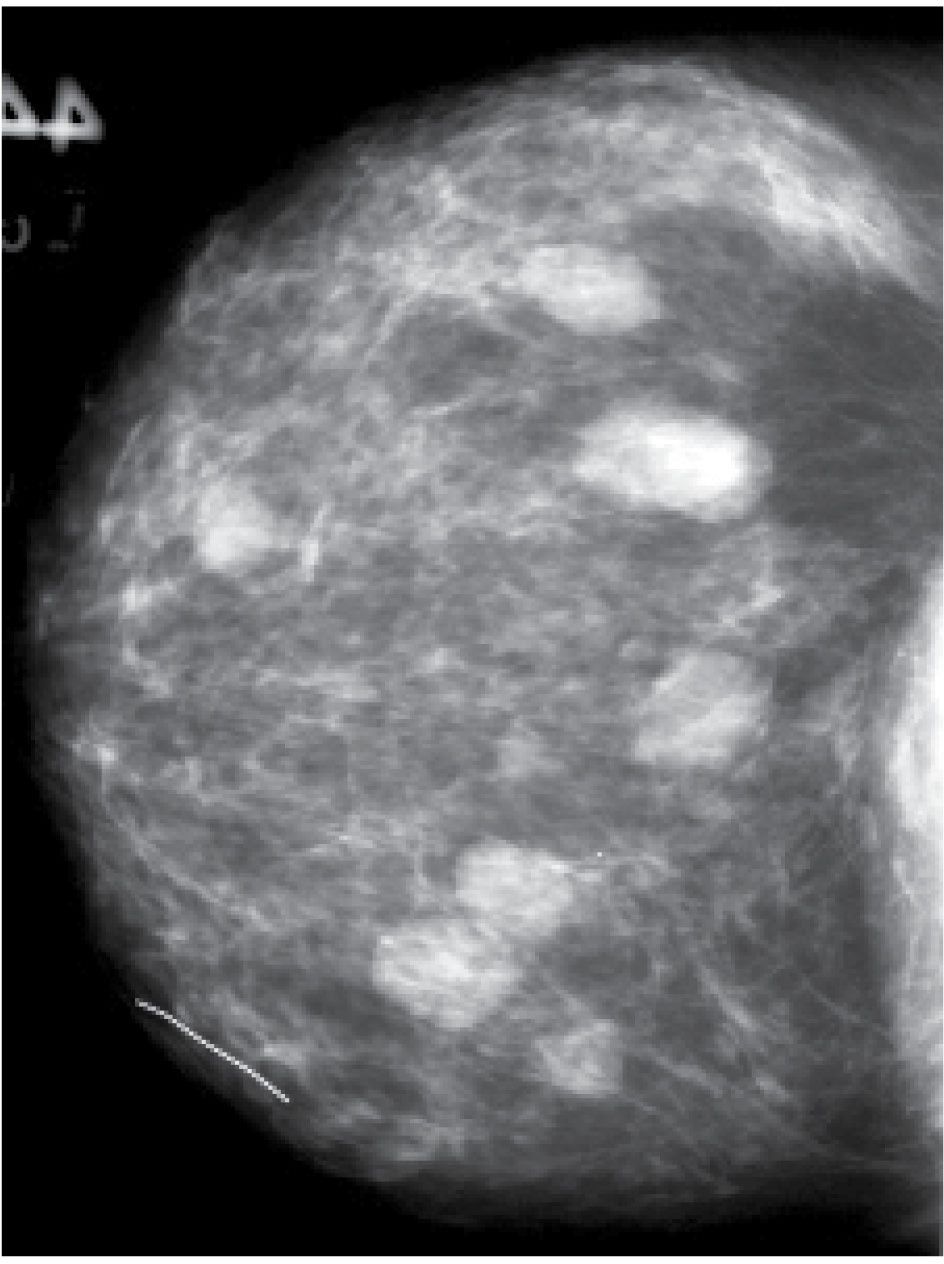
FIG. 7.5 • Fibroadenomas. Multiple, isodense, round, or oval masses with circumscribed margins randomly distributed in the right breast. BI-RADS 2: Benign findings.
Previous films are useful in the evaluation of masses. Although stability is not an absolute sign of benignity, if a mass with benign features (e.g., circumscribed margins, low to isodense) has been present for several years with no change, the likelihood of malignancy is low and recommending a 6-month follow-up is not appropriate. If the mass has features of malignancy (e.g., spiculation, distortion) that are not explained easily (e.g., prior trauma or surgery correlating with the site of the mass) it may represent a low-grade invasive lesion and biopsy is indicated (Fig. 7.6). In selecting prior films for comparison, try to use films from at least 2 years previously if available and, when evaluating a possible finding, look at multiple prior studies to establish slow progression or fluctuation in the finding. Subtle changes may not be apparent from 1 year to the next but may be striking when comparison is made with studies from 2 or 3 years previously (see Fig. 11.9).
Table 7.4 DIFFERENTIAL: MULTIPLE MASSES WITH SIMILAR FEATURES
Cysts Fibroadenomas Lymph nodes Papillomas Skin lesions (neurofibromas) PASH Invasive ductal carcinoma (multifocal or -centric) Peripheral papillary carcinomas Metastatic disease |

FIG. 7.6 • Tubular carcinoma. A: Spot compression view (only one projection shown) of the left breast demonstrates an oval dense mass (arrow) with spiculated margins in a 77-year-old patient with a history of a right mastectomy 25 years ago for breast cancer. B: Spot compression view, 12 years before the image shown in part A. No definite change in the mass (arrow) is noted; however, the patient has no history of surgery or trauma to this area. Tubular carcinoma or well-differentiated ductal carcinoma NOS is considered prospectively. BI-RADS 4C: Suspicious abnormality, biopsy is indicated. Tubular carcinoma with associated low nuclear grade DCIS with a cribriform pattern is diagnosed histologically. Sentinel lymph node biopsy is negative. A mass with spiculated margins or distortion that cannot be explained (e.g., trauma or surgery to that specific site) warrants biopsy in spite of stability on the mammogram. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
SKIN MASSES
Masses on the skin include moles, seborrheic keratosis, accessory nipples, skin tags, sebaceous cysts (epidermoid inclusion cysts), and neurofibromas (9). Keloids may also be noted projecting on the breast parenchyma. Unlike parenchymal masses, a thin lucency (air) may be noted surrounding the margins of the mass that protrude beyond the skin; the lucency is lost where the lesion attaches to the skin (Fig. 7.7). In some women, talc, calcifications, or air outlines the crevices of moles (Fig. 7.8A, B; also see Fig. 6.10). We mark skin lesions with a metallic BB prior to taking films so that we do not call a patient back unnecessarily for a skin lesion.
Sebaceous cysts and epidermoid inclusion cysts are common, often palpable, intradermal masses that may undergo changes in size from one year to the next. They commonly develop in the axilla (Fig. 7.9) or in the lower inner quadrants at the medial most extent of the inframammary fold (Fig. 7.10). On physical examination, sebaceous cysts may cause a smooth skin bulge and the orifice of the gland may be visible as a punctum (“black head”). On palpation, the mass moves with the skin such that you cannot glide skin over the mass. If squeezed, a white, thick, cheesy material that may be malodorous can be expressed from the punctum. When inflamed, localized erythema may be noted and tenderness elicited clinically; incision and drainage may be needed for treatment. However, complete removal of the cyst wall is required, otherwise, these lesions recur. On the mammogram, these masses have circumscribed margins or, when inflamed, indistinct (Fig. 7.11A) or spiculated (Fig. 7.12A) margins; associated calcifications may be present (Fig. 7.13; also see Fig. 6.9). On spot tangential views, they are localized to the skin (Figs. 7.9B and 7.12B). On ultrasound, an anechoic, hypoechoic, or echogenic mass often with posterior acoustic enhancement may be seen separating the dermal layers (Fig. 7.11C). As the patient is scanned and the transducer is manipulated, the skin track can be identified in some patients (Fig. 7.12B; also see Fig. 4.11). As the mass enlarges, the deep dermal layer may not be readily apparent (Fig. 7.10B). With inflammation or associated calcifications, the echotexture may be heterogeneous. These lesions can attain significant sizes such that patients present with discomfort particularly when the sebaceous cyst is in the axilla or along the inframammary fold (e.g., along the bra line). On magnetic resonance imaging (MRI), sebaceous cysts are masses localized to the skin that demonstrate a high T2 signal with no significant enhancement (Fig. 7.14), unless there is associated inflammation.

FIG. 7.7 • Skin lesions. Lucency (air) surrounding the portion of a lobulated mass that extends beyond the skin. The lucency (long arrows) is lost where the mass attaches to the skin (short arrows). Metallic BB is used to mark the skin lesion.

FIG. 7.8 • Seborrheic keratosis. A: Seborrheic keratosis (arrow) with encrusted high-density material and air outlining the crevices of the lesion. B: Seborrheic keratosis (arrow) with air outlining the interstices of the mass. BI-RADS 2: Benign finding. Ideally, the technologist should mark and document obvious skin lesions so that callbacks for skin findings are minimized.
FIG. 7.9 • Sebaceous cysts. A: Left MLO view photographically coned to upper aspect of the image. A round, dense mass (arrow) is imaged in the axilla correlating to a “lump” described by the patient. B: Spot tangential view. A round dense mass with circumscribed margins is imaged associated with the dermis, focally thickened at this site. This is a nice illustration of how useful the spot tangential can be in localizing lesions to the skin. BI-RADS 2: Benign finding. Sebaceous cysts can occur anywhere on the breast but are most common in the axillae and the medial most extent of the inframammary fold. If on physical examination a punctum is identified and the “lump” moves with the skin, and on the spot tangential view, the lesion is imaged in the skin, no further evaluation or short-interval follow-up is indicated. It is important to reassure the patient of the unequivocally benign etiology of the “lump.” If she is symptomatic, surgical referral for complete excision of the cyst wall/lining is appropriate.

FIG. 7.10 • Sebaceous cyst. A: Left CC view demonstrates a dense, oval mass with circumscribed margins at the medial most extent of the inframammary fold corresponding to the site of a “lump” described by the patient. The metallic BB marks the site of the palpable finding. B: Ultrasound. On visual inspection, a bulge in the contour of the breast is apparent at the site of the clinical finding. On physical examination a hard mass is palpated corresponding to the site of the mammographically described mass. An oval, lobulated complex cystic and solid mass with circumscribed margins and minimal posterior acoustic enhancement disrupting the deep dermal layer and extending into the subcutaneous tissue is imaged corresponding to the clinical and mammographically apparent mass. This is a sebaceous cyst. BI-RADS 2: Benign finding. Given the predilection of some of these lesions for the axillae and medial location along the inframammary fold (bra line), surgical excision is sometimes requested by the patient for symptomatic relief.
FIG. 7.11 • Sebaceous cyst. A: MLO view, photographically coned to the lower aspect of the breast in a patient who presents with a superficial “lump” that is painful. A metallic BB marks the location of the lesion. Increased density (arrows) is seen at the site of concern to the patient without a mass. B: Spot tangential view places the lesion (arrows) in tangent to x-ray beam and effectively localizes the finding to the skin. C: A hypoechoic mass (thick arrows) is imaged in the dermis. The mass splits the dermal layers such that the deep dermal layer (long arrows) is displaced inferiorly. BI-RADS 2: Benign finding. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 7.12 • Sebaceous cyst. A: Photographically coned down image in a patient who presents with a “lump” that is painful. An isodense, round mass with spiculated margins is imaged corresponding to the site of concern to the patient. Metallic BB used to mark the “lump” described by the patient. B: Ultrasound. A hypoechoic mass (thick large arrow) is imaged intradermally corresponding to the area of concern to the patient. A track to the skin surface is noted (small arrow). BI-RADS 2: Benign finding. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
Keloids are clinically apparent, developing at prior surgical sites. They occur with a higher incidence among Black and Hispanic patients and reflect abnormal wound healing. They are irregular, serpiginous, tubular, or mass-like structures noted at, and extending away from, the surgery site. The portion that projects beyond the skin is outlined by air such that a thin radiolucency is apparent mammographically (Fig. 7.15A). On MRI, they can be localized to the dermis and do not usually demonstrate significant enhancement (Fig. 7.15B). Treatment options are variable and include injections (steroids, 5 fluorouracil, interferon, retinoids, and calcium channel blockers) directly into the keloid, surgery, radiation therapy, topical applications of silicone gel, laser, pressure therapy, and cryosurgery. Mixed results are reported for each, and appropriate treatment remains controversial.

FIG. 7.13 • Sebaceous cyst with calcifications. A low to isodense, round mass with circumscribed margins and coarse calcifications internally. BI-RADS 2: Benign finding. Sebaceous cysts can develop calcifications that are commonly coarse, dense, and dystrophic in appearance.

FIG. 7.14 • Sebaceous cyst. MRI, sagittal T2 fat-suppressed image of the right breast. A mass (arrow) with smooth margins, high T2 signal, and no enhancement (images not shown) is imaged along the inframammary fold associated with the skin consistent with a sebaceous cyst.

FIG. 7.15 • Keloid. A: Right MLO view. A wide, tubular, low density, serpiginous structure (arrows) with a lucency (air) sharply defining some of the margins is seen involving the superior aspect of the right breast. Focal skin thickening is evident anteriorly at the inferior extent of the keloid. B: MRI, axial images postcontrast in the same patient. Masses (arrows) with smooth margins are seen associated with the skin. Minimal enhancement is evident on the subtraction images and on kinetic curve evaluation (not shown).
Clinically, neurofibromas are readily apparent in patients with neurofibromatosis. There is wide variation in the number, size, and distribution of the lesions on the breasts; however, there is a predilection for the periareolar areas in many patients. Although patients can develop new lesions, and preexisting lesions can increase in size, the rapid growth of a single lesion is of concern and biopsy may be indicated since malignant peripheral nerve sheath tumors may develop in preexisting neurofibromas (Fig. 7.16A). Neurofibromas are noted mammographically as masses that may be lobulated with circumscribed margins and partially or completely outlined by air. Depending on the number of lesions, evaluation of the underlying breast parenchyma may be difficult. Extra care should be used to actively disregard the obvious benign findings in search of a possible unsuspected malignancy in these patients. On ultrasound, a hypoechoic mass with gently lobulated, circumscribed margins and posterior acoustic enhancement is imaged in the dermis or in a subcutaneous location (Fig. 7.16B). On MRI, the lesions can be seen extending beyond the borders of the breast and usually demonstrate no significant enhancement (Fig. 7.16C).

FIG. 7.16 • Malignant peripheral nerve sheath tumor in a patient with neurofibromatosis. A: CC views. Multiple neurofibromas are evident bilaterally primarily involving the subareolar areas. Some of the neurofibromas project beyond the breast such that a thin lucency (air) can be seen surrounding the portion of the neurofibroma that extends beyond the skin. A neurofibroma (arrow) in the lower central aspect of the right breast posteriorly developed within a year and, as described by the patient, is “getting bigger, fast.” B: Ultrasound. A hypoechoic, macrolobulated mass with circumscribed margins and posterior acoustic enhancement is imaged in the subcutaneous tissue associated with the deep dermal layer. Because of the progressive change in size, an ultrasound-guided biopsy is done, and a malignant peripheral nerve sheath tumor is diagnosed. C: MRI, MIP image. Variably sized neurofibromas (arrows) with no enhancement are seen extending beyond the skin anteriorly from the periareolar margin as well as centrally in the cleavage.
An increasing number of patients are being referred to us for the evaluation of cellulitis. In most patients, no underlying etiology is identified; in some, radiation therapy may precede the presentation. Clinically, localized or diffuse erythema is the primary finding, and depending on the amount of inflammation, peau d’orange changes or localized areas of necrosis may be apparent (Fig. 7.17A); significant tenderness is elicited and often precludes a mammogram. The main differential consideration in many of these patients is inflammatory breast carcinoma (IBC). Patients with cellulitis are more likely to have localized findings, be more tender, and respond to antibiotics with no progression or recurrence of symptoms. At presentation, ultrasound is often the starting point because some of these patients do not tolerate the compression required for a mammogram. Ultrasound is helpful in localizing the process to the skin (Fig. 7.17B), excluding the presence of an underlying abscess requiring drainage or other focal parenchymal finding. Axillary lymph nodes may be prominent in patients with cellulitis; however, the lymph nodes retain normal morphologic features in contrast to the grossly abnormal lymph nodes that are seen in many patients with IBC at the time of presentation.
FAT-CONTAINING MASSES
Fat-containing masses in the breast are almost always benign (1–3,5). These masses can be completely fatty (Table 7.5) or mixed in density (Table 7.6). Although ultrasound findings are described for completeness, the diagnosis is established reliably when lucent or mixed-density masses are seen on the mammogram (with some of these lesions, the features on ultrasound may raise concerns inappropriately). It is important to note that, although many of the entities described are commonly fatty or mixed in density, they may also present as water density masses. So you will find an overlap in the differentials provided for each. For example, lymph nodes and fat necrosis are typically considered mixed-density masses, but each may present as a water density mass. Rarely, entities typically presenting as water density masses (including malignancy) may be noted to have lucent areas.
FIG. 7.17 • Cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection. A: Patient presents describing a red swollen left breast. The left breast is enlarged and diffusely erythematous with several areas of necrotic skin (eschar) medially. Skin tags are incidentally noted on the upper aspect of the abdominal wall and left axilla. B: Ultrasound. The dermis is thickened (short arrows), expands into, but does involve, the parenchyma and demonstrates a heterogeneous echotexture with bright echogenic foci (long arrows) possibly reflecting air. This process is limited to the skin and does not extend into the parenchyma; no solid or cystic masses are apparent in the breast tissue. The clinicians are told that the process is limited to the skin and is consistent with a cellulitis. MRSA is cultured; the patient is treated effectively with superficial debridement and IV antibiotics.
Table 7.5 DIFFERENTIAL: FAT DENSITY MASSES (RADIOLUCENT)
Lipoma Oil cyst Galactocele |
Table 7.6 DIFFERENTIAL: MASSES WITH MIXED DENSITY
Intramammary lymph nodes Fibroadenolipomas (hamartomas) Fat necrosis, oil cysts Galactocele Postoperative/traumatic fluid collections (hematomas, seromas) |
LIPOMA
Patients with a lipoma can be asymptomatic or present with a soft or hard, mobile mass. A radiolucent mass with an expansile circumscribed margin and a thin fibrous capsule are detected in the breast (Fig. 7.18A), or less commonly in the pectoral muscle (Fig. 7.18B), on the mammogram. Although the diagnosis is reliably made on mammographic findings, a mass with circumscribed margins and a homogeneously hypo-, iso-, or hyperechoic echotexture is imaged on ultrasound (see Fig. 4.40A) (10); in some patients, short, curvilinear hyperechoic internal septations may be apparent (Fig. 7.19A). Gentle mass effect can be seen on surrounding structures (Fig. 7.19B). Uncommonly, hemorrhage may occur in preexisting lipomas so that on the mammogram a mixed-density mass (Fig. 7.20A, B) is seen at the site of a preexisting lucent mass. As the hemorrhage resolves, the lucent nature of the mass becomes more apparent on follow-up mammograms; the mass is complex on ultrasound and fluid–fluid levels may be seen (Fig. 7.20C). A mass with the signal characteristics of fat (high T1 and T2 signal, suppressed on fat-suppression images) and no enhancement is incidentally noted on MRIs performed as screening studies in high-risk women or those being evaluated for other breast-related issues (Fig. 7.21). Histologically, these lesions are characterized by the presence of mature lipocytes surrounded by a thin capsule (11,12). If otherwise asymptomatic, no intervention or short-term follow-up is indicated. Rarely, liposarcomatous lesions can present in the breast typically as a rapidly growing mass. The size, age of the patient, and growth pattern should suggest a malignant process. On the mammogram, internal septations may be seen in an otherwise lucent mass and the typically homogenous echotexture on ultrasound may be more heterogeneous (see Fig. 8.50).
OIL CYSTS
Oil cysts are radiolucent, solitary (Fig. 7.22), or multiple (see Fig. 6.26B), uni- or bilateral masses. They vary in size, can progressively decrease, and resolve completely on subsequent mammograms. They are idiopathic or develop in areas of prior trauma or surgery and, in this setting, probably represent an end stage of fat necrosis. Some develop mural calcifications resulting in lucent-centered (or rim) calcifications (see Figs. 6.26B and 6.29A), while in others, the mural calcifications are seen “en-phase” having a coarse, irregular, curvilinear appearance (see Fig. 6.28). In most women, oil cysts are noted incidentally on screening mammography. Some patients may present for diagnostic mammography describing a discrete, hard mass that is correlated with one or multiple oil cysts mammographically. In this situation it is important to assure the patient and referring physician that the palpable finding is an oil cyst requiring no intervention or follow-up. The diagnosis is established on the mammogram (Figs. 7.23 and 7.24) such that ultrasound is rarely indicated. Given the variability in the appearance of oil cysts on ultrasound, the ultrasound features may raise concerns over the benign diagnosis (see Fig. 4.34). They may be anechoic with through-transmission indistinguishable from fluid-containing cysts (Fig. 7.23B). Internal echoes, fluid–fluid levels, septations, and complex cystic masses either predominantly cystic with intracystic or mural solid-appearing components (Fig. 7.24B) or solid with cystic components or they may appear solid (13). On MRI, oil cysts are characterized by circumscribed margins and demonstrate decreased signal on T2-weighted, fat-suppressed images (Fig. 7.25A) and a high signal on T2- and T1-weighted, non–fat-suppressed images. Most oil cysts demonstrate no enhancement (Fig. 7.25B); however, a thin enhancing rim may be apparent in some patients.
FIG. 7.18 • Lipomas. A: MLO views. A round, lucent mass with circumscribed margins is imaged in the right breast corresponding to the site of a “lump” described by the patient. The metallic BB denotes the palpable finding. Lucent masses in the breast are benign, and no further workup is indicated. BI-RADS 2: Benign finding. B: MLO views, different patient. An oval, lucent mass (arrows) with circumscribed margins is partially imaged in the left pectoral muscle. The size of the pectoral muscles is asymmetric, resulting in an asymmetric breast size as well. This is an intrapectoral lipoma that requires no additional evaluation. BI-RADS 2: Benign finding.

FIG. 7.19 • Lipomas. A: Ultrasound. An oval mass (calipers) with heterogeneous internal echotexture, predominantly hyperechoic with short, curvilinear foci of hyperechogenicity is correlated to a soft mobile lucent mass mammographically (mammogram not shown). BI-RADS 2: Benign finding. B: Ultrasound, different patient. A homogeneously hyperechoic mass (arrows) with short, curvilinear foci of hyperechogenicity and gentle mass effect on the pectoral muscle. BI-RADS 2: Benign finding.
FIG. 7.20 • Lipoma with hemorrhage. A: Left CC view. An oval lucent mass (arrows) is present centrally in the right breast. B: Left CC view following a motor vehicle accident. A mixed-density mass with circumscribed margins is now apparent at the site of the preexisting lipoma. BI-RADS 2: Benign finding. C: Ultrasound. A complex cystic mass with a fluid–fluid level is imaged corresponding to the mixed-density mass seen mammographically. Two years following the accident, the internal density had resolved, leaving the preexisting lucent mass.
FIG. 7.21 • Lipoma. A: MRI, sagittal, T1-weighted, nonfat-suppressed image of the left breast. An oval, fat containing mass (arrow) with circumscribed margins is imaged abutting the right pectoral muscle. B: MRI, T1 fat-suppressed sagittal image of the left breast postcontrast. The mass (arrow) is low in signal on the fat-suppressed images and demonstrates minimal enhancement of a thin rim. BI-RADS 2: Benign finding. The MRI was done for screening purposes in a high-risk patient with a personal history of breast cancer.

FIG. 7.22 • Oil cyst. Oval, lobulated, lucent mass (arrows) with circumscribed margins. BI-RADS 2: Benign findings.
In some women, oil cysts may be more appropriately characterized as mixed-density masses (Fig. 7.26) because of thickened, ill-defined, or spiculated margins, or the presence of a round or oval intracystic mass. With a history of trauma or surgery, and if fat (radiolucency) is associated with these masses in orthogonal projections, no intervention or short-term follow-up is warranted regardless of the spiculated margins or associated nodules (see below, fat necrosis).
FIG. 7.23 • Oil cyst. A: Photographically coned image of the right breast. An oval lucent mass (arrow) with circumscribed margins is identified. This is pathognomonic of an oil cyst with the diagnosis reliably established on the mammogram. BI-RADS 2: Benign finding. B: Ultrasound. An anechoic mass (arrow) with some posterior acoustic enhancement is imaged corresponding to the oil cyst seen on the mammogram. In this patient the ultrasound is not needed to reliably establish a benign diagnosis. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 7.24 • Oil cyst. A: Photographically coned image of the left breast in a patient who presents describing a “lump.” The metallic BB at the upper right hand corner of the image denotes the palpable finding. An oval lucent mass (thick arrow) with circumscribed margins is imaged corresponding to the palpable finding. Several round lucent masses (small thick arrows) are localized within the dominant mass on orthogonal projections (only one shown here). A dystrophic calcification is incidentally noted (long thin arrow). BI-RADS 2: Benign finding. B: Ultrasound. A complex cystic mass with mural nodules (arrows) is imaged corresponding to the palpable finding and the radiolucent mass seen on the mammogram. The ultrasound findings may raise concerns and differentials that include malignant processes; however, the mammographic findings effectively exclude those entities. In this patient, the ultrasound is not needed to establish a benign diagnosis accurately. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 7.25 • Oil cyst. A: MRI, sagittal T2-weighted fat-suppressed image of the left breast. An oval mass (arrow) with fatty tissue internally is imaged in the subareolar areas. B: MRI, axial image of the left breast following contrast. A fat-containing mass (arrow) with no associated enhancement is imaged at the site of the mass depicted in part A.
FIG. 7.26 • Oil cysts. Spot tangential view done at the site of a new “lump” (metallic BB) described by the patient in the right breast. Two adjacent oil cysts (long arrows) with thickened indistinct margins (or is it a mixed-density mass with central lucency?) correlating to the site of the palpable finding. A third oil cyst (small arrow) with no surrounding density is present. Note focal skin thickening and associated soft tissue stranding correlating to the location of the oil cysts. The findings are posttraumatic in etiology, and our job is to obtain correlative history to confirm this impression and reassure the patient of the benign etiology of the findings. Even though the margins are indistinct, the presence of fat centrally is sufficient to characterize this as a benign finding. No additional intervention of follow-up is indicated for this finding. BI-RADS 2: Benign finding.
Steatocystoma multiplex is a rare condition with an autosomal dominant mode of inheritance associated with multiple intradermal oil cysts scattered over the body but with a predilection for the trunk and upper extremities (14,15). Although more commonly seen in males, women with this condition may be found to have multiple oil cysts in the breasts bilaterally.
MIXED-DENSITY MASSES
Lymph Nodes
Intramammary and axillary lymph nodes are common and variable in number, size, density, shape, and location (Fig. 7.27). They can fluctuate in size, and in some women can disappear only to reappear on subsequent studies. Keep in mind that on the basis of the positioning of the woman for the MLO views, you may see variable amounts of axillary tissue, and with that, a variation in our ability to evaluate axillary lymph nodes mammographically. Typically, they are round or oval masses of mixed density with circumscribed and sometimes lobulated margins located in the upper outer quadrants posteriorly and in the axillae; however, they can be found anywhere, including, less commonly, the medial quadrants of the breast. The presence of a variably sized fatty hilum either centrally or peripherally is required prior to assuming that a mass in the upper outer quadrant of the breast is a lymph node. Size alone is not used to determine the need for evaluation. We rely on density, margins, contour (bulging) alterations, absence of a fatty hilum, and changes compared with prior studies to determine appropriate management.
On ultrasound, lymph nodes are round or oval hypoechoic masses with circumscribed margins and an area of hyperechogenicity either centrally or peripherally (Fig. 7.28; also see Fig. 4.21). Reactive lymph nodes may demonstrate a symmetric increase in the size and density of the cortex and may or may not retain a fatty hilum. If the fatty hilum is not readily apparent on the mammogram, it may be seen on ultrasound (Fig. 7.28); however, if a fatty hilum is not identified with either modality and the mass is new or enlarging, biopsy is indicated (Fig. 7.29). Although in evaluating lymph nodes it is important to factor in all features including cortical thickening and bulging (16), the echogenicity of the cortex on ultrasound can be a helpful guide. The cortex in patients with an underlying inflammatory process is often iso to slightly hyperechoic, whereas in patients with metastatic breast cancer or lymphoma, the cortex is often markedly hypoechoic (almost anechoic), the fatty hilum is either attenuated or not present, and there may be posterior acoustic enhancement (see Figs. 4.22, 8.59, through 8.61).

FIG. 7.27 • Lymph nodes. A: MLO views. Multiple masses (arrows) with circumscribed margins are present in the axillae extending into the parenchyma bilaterally. They are variable in size, shape, and density (within and among patients). Some are mixed in density. B: MLO views, different patient. Variably sized, mixed-density masses (arrows) in the axillae. It is important to emphasize that size alone is not useful in assessing lymph nodes in the axillae.
FIG. 7.28 • Lymph node. A: Spot compression views (only one is shown) of the upper outer of the right breast demonstrate an oval isodense mass with circumscribed margins. No identifiable fatty component is seen. Additional evaluation with ultrasound is indicated. B: Ultrasound. An oval mass (calipers) with circumscribed margins and an echogenic fatty hilum is identified along the 10 o’clock axis, 6 cm from the right nipple corresponding to the mass seen mammographically. Note that the cortex is slightly hyperechoic. BI-RADS 2: Benign finding. Although intramammary lymph nodes are most commonly seen in the upper outer quadrants of the breast as in this patient, they can be found anywhere, including the inner quadrants in approximately 5% of women.

FIG. 7.29 • Lymph node, reactive. A: Spot tangential view of a “lump” described by a 70-year-old patient. A round, iso-to dense mass with circumscribed margins is present with an associated partial “halo” (arrows). This mass is not identified on prior studies. Additional evaluation with ultrasound is indicated. B: Ultrasound. A round hypoechoic mass with circumscribed margins and minimal posterior acoustic enhancement is imaged correlating to the palpable and mammographically apparent mass. No fatty component is identified with either modality. A new mass that is solid requires biopsy, particularly in a 70-year-old patient. BI-RADS 4B: Suspicious finding, biopsy is indicated. A reactive lymph node is described on the ultrasound-guided core biopsy.
The oval shape and circumscribed margins of lymph nodes described mammographically and on ultrasound are also noted on MRI (see Fig. 5.18). The distribution and relationship to vasculature is readily appreciated on MRI. The extension of lymph nodes from the axilla inferiorly along the midaxillary line is also readily apparent on MRI in some patients (see Fig. 8.61A). The enhancement pattern of lymph nodes is variable but normal lymph nodes commonly demonstrate rapid washin and washout kinetics on the dynamic T1 sequences. Unlike most malignancies, lymph nodes demonstrate an intermediate to high T2 signal. The fatty hilum may have a high signal on T1 non–fat-suppressed sequences and low signal on the fat-suppressed sequences.
On screening mammograms, diffuse increases in the size and density of axillary, and less commonly intramammary, lymph nodes may be identified in some patients. Before undertaking extensive workups it is important to review the patient’s history with respect to possible underlying causes of benign lymphadenopathy (Table 7.7) (17). If no underlying etiology is readily identified in the patient’s history, the patient is called back and evaluated with spot compression views, physical examination, and ultrasound. On the basis of this evaluation, a core biopsy may be done (Fig. 7.30) to determine the underlying cause of the adenopathy (18).
Gold particles imaged as high density, punctate particles mimicking microcalcifications can be seen bilaterally in the axillary and intramammary lymph nodes of women treated for rheumatoid arthritis with gold (Fig. 7.31) (19). Coarse calcifications occurring in lymph nodes are often related to granulomatous disease and do not require intervention.
In a retrospective 5-year review, Lee et al. (20) reported unilateral enlargement of axillary or intramammary lymph nodes in 0.2% of their patients with otherwise normal mammograms. In their experience, biopsy is indicated in patients with a history of an underlying malignancy if the lymph node enlarges by more than 100% over baseline. If the woman does not have a history of a malignancy, the lymph node enlargement is small, the node is not palpable, and it maintains a benign appearance, they suggest clinical and mammographic follow-up. In the majority of their patients, lymph node enlargement decreased on follow-up studies. Please see Chapter 8 for a more detailed discussion of the imaging features of potentially abnormal lymph nodes.
Fibroadenolipomas (Hamartoma)
Fibroadenolipomas (FAL) or hamartomas are characterized by the presence of a pseudocapsule within which fatty, glandular, and fibrous elements are admixed. This appearance has led some to describe them as a “breast within a breast” (Fig. 7.32A). The overall density of these lesions is variable depending on the proportions of intermingled fat and glandular tissue. In some women, FAL may enlarge and present as a palpable mass (Fig. 7.33A). Since the lesions are made up of breast tissue, breast cancer of any type can arise in hamartomas (21). Development of pleomorphic calcifications (Fig. 7.34), increasing density, particularly if ill-defined or spiculated in a FAL, should prompt further evaluation and, if indicated, an imaging-guided biopsy. If the patient presents with a palpable mass in an FAL, imaging guidance is helpful in targeting the areas of soft tissue in the FAL; otherwise, false-negatives may result if fatty elements are sampled. On ultrasound, these lesions can be distinguished from the surrounding glandular tissue and have a heterogeneous echotexture with admixed areas of hypo- and hyperechogenicity; gentle mass effect may be seen on the surrounding tissue (Fig. 7.33B). On MRI, fatty and glandular elements are isolated within the breast by a thin “pseudocapsule” (Fig. 7.32B). Progressive enhancement of the glandular tissue may be seen on the dynamic sequence (Fig. 7.32C).
Table 7.7 BENIGN CAUSES OF INTRAMAMMARY AND AXILLARY LYMPHADENOPATHY
Lymphoid hyperplasia (acute or chronic inflammation) Collagen vascular disorders (rheumatoid arthritis, scleroderma, lupus) Granulomatous disease (sarcoid, tuberculosis) Human immunodeficiency virus (HIV) Dermatopathic (exfoliative or atopic dermatitis, psoriasis, infectious rashes) Silicone adenopathy Histoplasmosis Toxoplasmosis (cat scratch) |

FIG. 7.30 • Lymph nodes, HIV. A: MLO views from a screening study photographically coned to the upper portion of the images. Multiple, dense, round, and oval lymph nodes with circumscribed margins are imaged in the axillae. These findings represent a change compared with prior studies in a patient with no significant medical history. BI-RADS 0: Need additional imaging evaluation. B: Ultrasound. Multiple lymph nodes are imaged in the axillae; this image of a lymph node in the right axilla is representative of their features. All of the lymph nodes have a fatty hilum and thickened cortices that are iso to slightly hyperechoic but exert no mass effect on the hyperechoic fatty hila. Findings on core biopsy are suggestive of HIV, a diagnosis that is confirmed on blood tests.
FIG. 7.31 • Gold deposits. A: High-density particles in an axillary lymph node (arrows) simulate calcifications. This is gold in a patient with rheumatoid arthritis. B: Different patient. High-density particles in two axillary lymph nodes (arrows). This is gold in a patient who received gold treatments for rheumatoid arthritis. Gold is typically seen as high-density lace-like particles involving all otherwise morphologically normal intramammary lymph nodes and those in the axillae. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 7.32 • Fibroadenolipoma and invasive ductal carcinoma. A: CC views from a screening study. A mixed-density mass (short arrows) is imaged in the right subareolar area. Don’t be mesmerized by the obviously benign finding, force your brain to focus away from the benign mass in search of potential cancers. A round mass (long arrow) is present approximately 1.5 cm posterior to the fibroadenolipoma. On spot compressions (not shown) it is characterized by spiculated margins; an invasive ductal carcinoma is diagnosed on an ultrasound-guided core biopsy (not shown). B: MRI, sagittal T2-weighted fat-suppressed image. The fat signal in the FAL (arrows) is suppressed, and the glandular tissue demonstrates intermediate T2 signal. C: MRI, axial T1-weighted image postcontrast. A round, rim-enhancing mass (long arrow) with circumscribed margins is seen in the central aspect of the right breast correlating with the mass seen mammographically and the site of the patient’s known invasive ductal carcinoma. The fibroadenolipoma (short arrows) is noted in the right subareolar area. The glandular elements in the FAL are characterized by slow to medium washin and persistent-delayed kinetic curves.
FIG. 7.33 • Fibroadenolipoma. A: Photographically coned view of the anterior aspect of the right breast in a patient who presents describing a “lump.” A metallic BB denotes the location of the “lump.” A fat containing mass is imaged mammographically. Predominantly fatty tissue with some islands of glandular tissue surrounded by a fibrous pseudocapsule (arrows) is imaged correlating to the site of the palpable finding. The benign etiology of the finding is made based on the mammographic finding. BI-RADS 2: Benign finding. B: An oval hyperechoic mass (arrows) with areas of hypoechogenicity is seen on ultrasound correlating to the palpable and mammographically apparent mass. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 7.34 • Fibroadenolipoma with developing DCIS. A: Photographically coned view of the anterior aspect of the right breast in a 64-year-old woman who is asymptomatic. A mixed-density mass (arrows) with predominantly dense glandular but some intermingled fatty tissue is noted consistent with a hamartoma or FAL. BI-RADS 2: Benign finding. B: Subsequent screening mammogram demonstrates calcifications (arrow) developing in the FAL. BI-RADS 0: Need additional imaging evaluation. Magnification views are indicated. C: Spot compression magnification views (only one projection is shown) demonstrate grouped fine linear calcifications (arrow). BI-RADS 4C: Suspicious abnormality, biopsy is indicated. A stereotactically guided biopsy is done. DCIS, high nuclear grade with central necrosis is diagnosed on the cores. The tissue in FALs is no different than tissue elsewhere in the breasts; it needs to be evaluated as meticulously as glandular tissue elsewhere in the breasts. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
Fat Necrosis
Trauma or surgery resulting in the release of fatty substances in the stroma of the breast can lead to an inflammatory response commonly characterized, in the acute setting, by an irregular mass with fatty tissue centrally, spiculated margins and architectural distortion; skin thickening and retraction may be present (Fig. 7.35). Alternatively, a round or oval mass with indistinct margins (Fig. 7.36A), an irregular mass with spiculated margins (Fig. 7.37A), a mixed-density mass with indistinct margins, or an irregular area of density (Fig. 7.38A) may be seen. As the inflammatory response subsides, the mammographic and ultrasound features of the lesion usually evolve. In some women, the mixed-density mass with spiculated margins becomes less dense and a fatty center develops; as the ill-defined soft tissue component resolves, a smooth thin walled oil cyst may be all that remains. The progression from dense, mass with spiculated margins to oil cyst is appreciated as films are viewed sequentially (Fig. 7.38B, C). In the intermediate stages, densities or nodules may be seen in the developing oil cyst (Fig. 7.38B, C). As the lesion continues to evolve, dystrophic-type calcifications can develop (Fig. 7.39); however, early in their formation, the calcifications can be linear with irregular margins and pleomorphism indistinguishable from the microcalcifications associated with comedo necrosis in ductal carcinoma in situ (DCIS) (see Figs. 6.43A and 6.46C, D). In other women, fat necrosis resolves completely, with no residual abnormality seen on subsequent studies (22). Less common appearances for fat necrosis include developing parenchymal asymmetry (Fig. 7.40A) or trabecular thickening, forming a fine reticular pattern (Fig. 7.40B).

FIG. 7.35 • Fat necrosis. Spot tangential view done at a lumpectomy site demonstrates a mixed-density mass, with central lucency, indistinct, and spiculated margins associated with skin thickening and retraction. BI-RADS 2: Benign finding.


FIG. 7.36 • Fat necrosis. A: MLO views in a 76-year-old patient with a history of left breast cancer treated with lumpectomy and radiation therapy 12 years ago. The left breast is smaller with distortion posteriorly at the lumpectomy site (metallic clip is seen at edge of image) and progressive asymmetrical calcification (long arrows) of the arteries likely a radiation therapy effect. A new round mass (short arrow) with indistinct margins is confirmed on spot compression views (not shown) in the superior aspect of the left breast. No internal fatty component is apparent mammographically. B: MRI, T1 axial postcontrast image. Oval mass (arrow) with peripheral enhancement characterized by rapid washin and washout type kinetic curves corresponding to the mass seen mammographically. Asymmetric breast size is again apparent with the left breast smaller. A homogeneously enhancing lymph node with smooth margins is noted in the right axilla. C: MRI, sagittal T2-weighted fat-suppressed image of the left breast. A mass with a low T2 signal consistent with a fat (arrow) is imaged at the site of the enhancing mass. Fat necrosis is confirmed on core biopsy. Edematous changes along the pectoral fascia are related to the prior lumpectomy and radiation therapy.

FIG. 7.37 • Fat necrosis. A: Spot compression view done to evaluate a screen-detected abnormality. An irregular mass with spiculated margins and distortion is confirmed on the spots (only one projection is shown). B: A vertically oriented mass with angular margins and intense shadowing is imaged in the upper inner quadrant of the right breast, zone B corresponding to the mammographic finding. On questioning, the patient recalls having had a fall one year ago with bruising of her right breast. The correlation with the clinical history is critical because otherwise this lesion requires biopsy. It is our contention that the correlation is best established by the breast imager. BI-RADS 2: Benign finding. The mammographic finding has resolved on follow up screening studies.
Soo et al. (23) describe a wide range of ultrasound patterns for fat necrosis. In their experience, a complex mass with echogenic bands that may shift in position as the patient is moved is strongly suggestive of fat necrosis. They also found masses with echogenic mural nodules (Fig. 7.41B) that evolve with time, solid-appearing masses, and anechoic masses with posterior acoustic enhancement or shadowing (Fig. 7.37B) as manifestations of fat necrosis on ultrasound. In our experience, one of the more common ultrasound features of fat necrosis is an area of hyperechogenicity that may be well- to ill-defined, associated with cystic spaces or small round or oval areas of hypoechogenicity (Fig. 7.41C; also see Fig. 4.36) commonly disrupting tissue planes.
FIG. 7.38 • Fat necrosis. A: Photographically coned down view of the left breast demonstrating an ill-defined mixed-density mass (arrows) correlating to a prior excisional biopsy site. B: Three months later. The mass (white arrow) is getting smaller and the central fatty component is increasing. A soft tissue component (black arrow) is apparent in the oil cyst. C: Four years later. The soft tissue component continues to decrease with only a small amount of residual soft tissue density remaining surrounding the oil cyst. Intracystic nodule is also almost completely resolved. BI-RADS 2: Benign finding. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 7.39 • Fat necrosis. Spot compression view demonstrates a mixed-density mass with indistinct and spiculated margins as well as dense, course dystrophic-type calcifications. Localized skin thickening and retraction are also apparent. BI-RADS 2: Benign finding.
FIG. 7.40 • Fat necrosis. A: Palpable area (metallic BB) of parenchymal asymmetry (arrows) developing at the site of chest wall trauma. Ultrasound (not shown) demonstrates disruption of normal tissue architecture with diffuse hyperechogenicity and associated small cystic spaces. B: Palpable area (metallic BB) of parenchymal asymmetry and prominence of the trabecular markings (white arrows) developing at a site of trauma. Some amorphous calcifications are present in this area (black arrow). (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 7.41 • Fat necrosis. A: Spot tangential view done at the site of a “lump” described by the patient in her right breast. Oval, mixed-density or fat containing mass with circumscribed and indistinct margins as well as associated dystrophic calcifications (arrow) corresponding to the “lump” described by the patient. The metallic BB is used to denote the site of the “lump.” The mass developed at a site of prior trauma. Given the history of trauma, and a correlating mixed-density mass mammographically, no further evaluation or short-interval follow-up is indicated. BI-RADS 2: Benign finding. B: Ultrasound. An oval complex cystic and solid mass with mixed shadowing and posterior acoustic enhancement is imaged correlating to the palpable and mammographic finding. C: Ultrasound, different patient. A hyperechoic mass (arrows) with internal cystic changes and indistinct margins is imaged correlating to the clinical and mammographic finding. Note the disruption of normal tissue planes. Complete resolution of this finding is noted a year later on the screening mammogram.
The appearance of fat necrosis on MRI is variable and, as seen with mammography and ultrasound, fat necrosis can mimic breast cancer on MRI (24). Low signal is noted on fat-suppressed T1 and T2 images (Figs. 7.36C and 7.42C) correlating with the fatty component seen mammographically, commonly in a subcutaneous location. The edges of these lesions may demonstrate smooth rim enhancement in the predominantly cystic lesions; however, the rim may be thickened, spiculated, or indistinct. If there is no lipid component, these lesions can simulate cancers morphologically with kinetic curves that can range from slow to rapid initial washin and progressive, plateau, and rapid washout delayed kinetics (Figs. 7.36B and 7.42B).
A history of trauma or surgery and the appearance of the lesion on sequential mammograms provide the assurance needed to characterize these lesions as benign in many patients. Ultrasound is usually not needed for the diagnosis. It is critical to recognize that following biopsy or trauma, mammographic findings peak approximately 4 to 6 months following the event, after which the findings stabilize or slowly resolve as described. Rarely, fat necrosis can develop and increase in size and density years after a biopsy or trauma. Since the mammographic, ultrasound (Fig. 7.43A, B), and MRI (Figs. 7.36B and 7.42B) features of fat necrosis overlap those of cancer, biopsy is indicated in those patients without a history of surgery or trauma correlated to the site of the imaging findings or in those in whom it develops years after the surgery with no apparent cause.

FIG. 7.42 • Fat necrosis. A: Spot compression view, right breast. An irregular mass with spiculated margins and associated distortion (arrows) is confirmed on the spot compression views (only one projection is shown). No correlative abnormality is identified on ultrasound at the expected location of the mammographic finding. B: MRI, sagittal maximum intensity projection image. An irregular mass (short arrow) with spiculated margins, distortion, and heterogeneous enhancement characterized by rapid washin and washout kinetic curves corresponding to the mass seen mammographically. Incidentally noted are multiple foci of nonspecific enhancement randomly scattered in the parenchyma and several enhancing lymph nodes (long arrows) with retained fatty hila. C: MRI, sagittal T2 fat-suppressed image of the right breast. Distortion with central low T2 signal (short arrow), suggestive of fat internally and high T2 signal spiculations is imaged corresponding to the enhancing mass and mammographic finding. Fat necrosis is diagnosed following an MRI-guided biopsy. Note masses (long arrows) with high T2 signal and fat-suppressed hila consistent with lymph nodes.
FIG. 7.43 • Fat necrosis. A: Ultrasound. Mass (arrows) with indistinct and spiculated margins and some shadowing corresponding to a new mass detected mammographically. Fat necrosis is diagnosed following an ultrasound-guided core biopsy. B: Ultrasound. One year later. A mass (arrows) with angular and spiculated margins, an echogenic rim, and intense shadowing is imaged at the site of the prior biopsy; it is now palpable. C: Spot tangential compression view done at the site of the “lump” described by the patient corresponding to the previously biopsied mass shown in parts A and B. Dense, round mass with indistinct and spiculated margins is imaged corresponding to the site of the palpable finding. The lesion has stabilized on subsequent mammograms.
Histologically, fat necrosis is characterized by acute changes that evolve with time. Initially, fat cell disruption and hemorrhage is followed by hemosiderin deposition, infiltration by variable numbers of histiocytes, plasma cells, and lymphocytes. Chronic, progressive fibrosis develops peripherally surrounding the necrotic fat cells and calcifications (11,12).
WATER DENSITY MASSES
These masses contain no fat. In addition to the shape and marginal features of these masses, consider their density. The density can be described as low, equal to, or increased, compared with the density of an equal volume of breast tissue. Differential considerations for the more common benign and malignant round or oval masses with circumscribed to indistinct margins are provided in Table 7.8. The differential for some of the more common benign and malignant masses with spiculated margins is provided in Table 7.9. Depending on history, clinical presentation, palpable findings, imaging features (mammography and ultrasound), the lists can be more appropriately focused to a given patient and the clinical and imaging features of the mass. The age of a patient is a particularly critical factor when considering an appropriate differential for an individual patient: a developing, solid mass in a postmenopausal patient raises differential considerations that are different from the considerations for a developing solid mass in a woman in her 30s or early 40s.
Table 7.8 DIFFERENTIAL: ROUND, OVAL (EXPANSILE) WATER DENSITY MASSES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree