So you found the architectural distortion on her screening mammogram. She had a diagnostic workup and you found a small abnormal area, no more than 1 or 2 cm. Ultrasound-guided core biopsy showed invasive lobular carcinoma (ILC). She opted for mastectomy even though she looked like a good breast conservation candidate. The pathologist found 7 cm of tumor! How could I have underestimated the size by so much? How could we not have seen such a big invasive cancer last year?
This scenario is actually quite common with ILC, which invades the breast in thin strands of cells much like a spider web. In this chapter we will review the less common breast cancers, including some pathology correlation that may help you expand your differential diagnosis beyond invasive ductal carcinoma (IDC).
Invasive ductal carcinoma–not otherwise specified (IDC-NOS) and ductal carcinoma in situ (DCIS) are by far the most commonly diagnosed breast cancers, making up about 85% of new cases. The remaining 15% of breast cancers often have features that suggest that the diagnosis is something less common.
IDC-NOS simply means that the cancer is differentiated enough to form ducts but does not display other differentiating features, such as making mucin (as in mucinous carcinoma) or papillary formations (as in papillary carcinoma). IDC-NOS typically presents as a spiculated mass or developing asymmetry. DCIS, the nonobligatory predecessor to IDC, typically presents as either coarse heterogeneous or fine pleomorphic calcifications.
The “other” breast cancers can be broadly divided into ILC, the well-differentiated subtypes of IDC, tumors of stromal origin, and metastatic carcinomas. Some rare types of breast malignancies also display characteristic features that suggest the diagnosis. An understanding of these less common breast malignancies will improve skills in detection and diagnosis.
Invasive Lobular Carcinoma
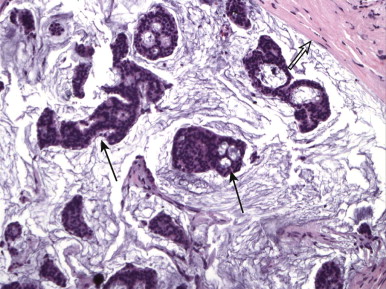
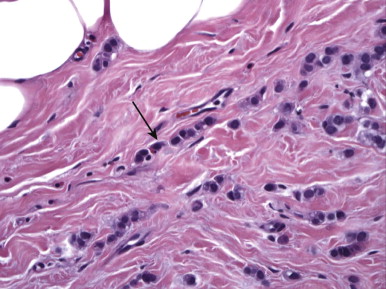
ILC is very different from IDC. On histologic examination, it is characterized by a lack of e-cadherin. Why are we boring you with pathology? Although the study of Latin among premedical students is less common these days, e-cadherin sounds rather like “adhere,” which explains ILC in a nutshell. The cells in ILC lose their ability to adhere to one another, resulting in cancers that are very infiltrative in the breast, like a spider web. ILC is characterized by lines and sheets of cells invading into the breast tissue ( Fig. 11-1 ). This insidious growth pattern and failure to elicit a desmosplastic reaction can make ILC difficult to detect on clinical examination and mammography.
Let’s think about how a spider web of tumor cells in the breast (ILC) is going to be different from IDC, where a mass of adherent tumor cells is more characteristic. How would a web of tumor cells feel on clinical examination? Although IDC typically presents as a firm palpable mass on clinical examination, ILC more often presents with an ill-defined mass or thickening of the tissue, or with nipple retraction if the web becomes tethered to the subareolar tissues. If the web of tumor cells is dense enough, ILC can also present as a firm palpable lump.
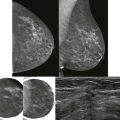
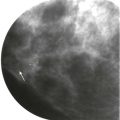
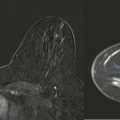
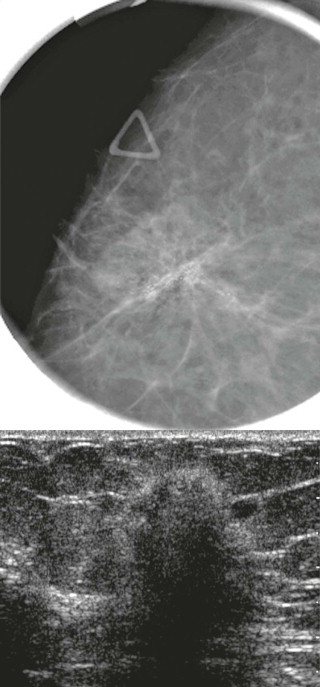
How would a web of tumor cells look on a mammogram or ultrasonography (US)? The mammogram may show only very subtle findings or be completely normal, even in the setting of extensive ILC ( Fig. 11-2 ). Only the densest part of the tumor will be visible on mammography. ILC may appear as a mass with ill-defined or spiculated margins or as architectural distortion with or without a central mass. When a central mass is present, then it is considered a spiculated mass. When there is architectural distortion without a central mass, these lesions are often called the “dark star.” Calcifications are uncommon, though ILC can have associated DCIS. ILC is often seen in only one view, most commonly the craniocaudal (CC) view. This makes sense because the CC view has better compression of the breast tissue than does the mediolateral oblique (MLO) view. Greater compression can bring out the subtle distortion to better advantage. The extent of disease of ILC is not easily assessed on mammography because the edges of that web are not well defined.
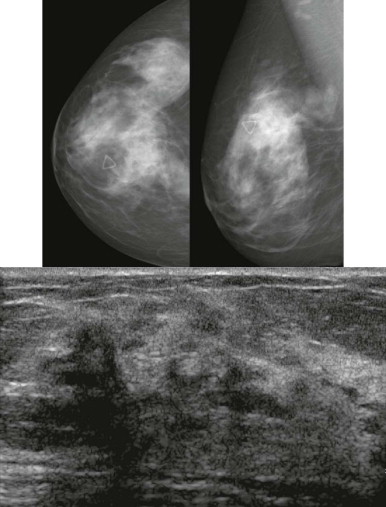
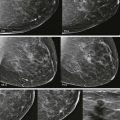
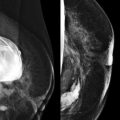
When the tumor is very large, the breast affected with ILC can appear to be getting smaller on mammography—the “shrinking breast” ( Fig. 11-3 ). This is not due to the breast becoming physically smaller, but to the decreased compressibility of the breast tissue that is full of webs of cancer cells. If the contralateral breast compresses to a thickness of 5 cm, a breast with extensive ILC may only compress to 8 cm. Although this results in the appearance of a smaller breast on mammography, breast size is typically symmetric on clinical examination.
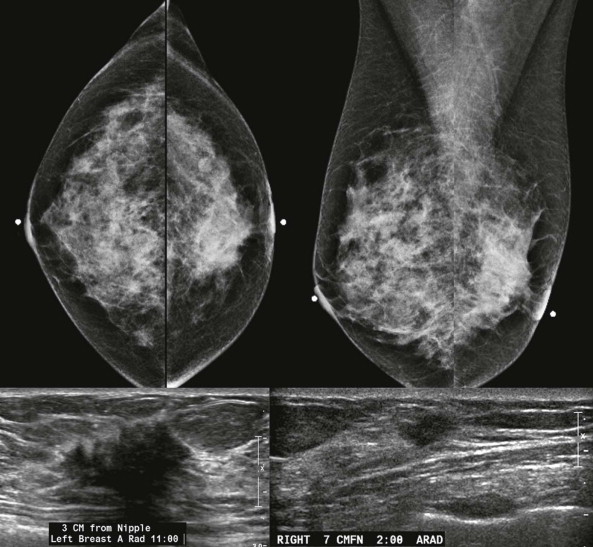
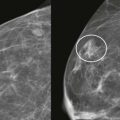
On US, ILC may present as a defined mass (see Fig. 11-3 ) but more often presents as ill-defined areas of shadowing without a distinct mass (see Fig. 11-2 ). The lines and arcs of tumor can produce bands of shadowing, like the edges of a spider web. Although tumor size is often underestimated by US, this modality is more accurate than clinical examination or mammography in assessing extent of disease.
Magnetic resonance imaging (MRI) is helpful in evaluating the extent of disease with ILC, showing more extensive disease than does mammography in 39% of cases. A cautionary note—ILC may show only faint enhancement with progressive kinetics on MRI. The washout pattern is much less common for ILC than IDC. Remember—when interpreting breast MRI and deciding on your management recommendation, the morphologic appearance always trumps the curve.
ILC is often larger at diagnosis than IDC and is often multifocal. Likewise, ILC is more commonly associated with positive margins and more often treated with mastectomy than IDC. Axillary metastasis is less common, however, so overall ILC has a similar prognosis to IDC. The most evil variant of ILC is pleomorphic ILC. These tumors show more variability of the nuclear size and less uniform cell size. A few recent studies suggest that pleomorphic ILC may have a higher predisposition to metastasis and a worse prognosis compared with usual ILC.
When ILC metastasizes, it may spread to strange places like peritoneal surfaces, the stomach, uterus, ovaries, and bladder. ILC may therefore present with ascites, hydronephrosis, or pelvic masses.
As might be expected, fine-needle aspiration (FNA) of ILC is less sensitive than for IDC. This is logical because FNA of ILC attempts to obtain material from single-file sheets of tumor cells with intervening normal tissue, rather than from a more easily targeted mass of adherent tumor cells that is seen with IDC.
Lobular carcinoma in situ (LCIS) is characterized by a monomorphic population of cells expanding lobules. It is typically mammographically occult and, as such, is an incidental finding on biopsy. However, proliferative lesions such as LCIS are common on biopsy of MRI-detected lesions.
Pleomorphic LCIS is characterized by increased cellular atypia compared with usual LCIS. Some small series suggest a higher incidence of associated invasive carcinoma when core biopsy shows pleomorphic LCIS ( Boxes 11-1 and 11-2 ).
- •
Prior to the mid-1990s, LCIS was treated aggressively as a premalignant condition, often resulting in bilateral mastectomy.
- •
In the mid-1990s, studies suggested that LCIS was not a premalignant neoplasm but a marker of high risk, elevating the risk of breast cancer four- to sevenfold in either breast, and for either ILC or IDC.
- •
Studies now show that LCIS and ILC have similar genetic mutations, suggesting that LCIS is indeed a premalignant lesion.
- •
LCIS appears to be a nonobligate precursor of ILC. The risk for subsequent invasion may be very low or delayed in onset compared with the risk of IDC developing from DCIS.
- •
IDC-NOS
- •
ILC
- •
Radial scar
- •
Surgical scar
Radial scar can mimic ILC on mammography. Both frequently present as distortion without a central mass (the “dark star”). Radial scar is not actually a scar; it is not due to trauma or surgery, but does look somewhat like a scar at histologic examination. Benign lobules and ducts are entrapped by dense central fibrosis and elastosis, which results in the appearance of architectural distortion on the mammogram ( Fig. 11-4 ). The cause is unclear, but these are proliferative lesions. Hyperplasia, atypical ductal hyperplasia, and papillomas are common in the surrounding tissue. From 10% to 30% of radial scars are associated with DCIS or IDC. Multiple small foci of carcinoma are not uncommon. A history of biopsy showing radial scar is also associated with a higher risk of breast cancer (relative risk of two times that of women without this history).
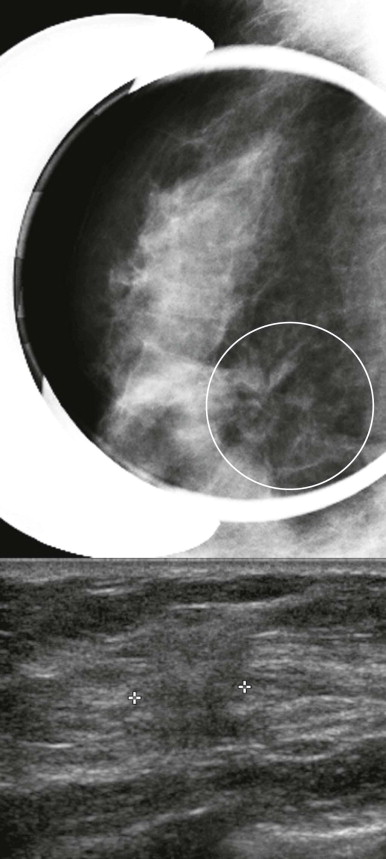
Radial scar may also mimic an invasive cancer, particularly tubular carcinoma, at both gross and histopathologic examination. A myosin stain is sometimes used to identify myoepithelial cells in the basement membrane, which will be present in radial scar but not in invasive carcinoma.
Radial scar is a general term that may refer to any of these pathologic entities: radial sclerosing lesion, sclerosing papillary proliferation , and complex sclerosing lesion . Radial scar/complex sclerosing lesion is characterized by a dense central fibrotic nidus surrounded by circumferentially radiating ducts and lobules. Complex sclerosing lesions are similar, but larger (usually > 10 mm) and less organized. Sclerosing papillary proliferation is a sclerosing lesion with associated papillomas.
Management of architectural distortion having the dark star appearance on mammography is somewhat controversial. Up to 29% of these women will have invasive carcinoma. At initial mammographic interpretation, either core biopsy or diagnostic surgical biopsy can be performed. Core biopsy showing a benign result will often result in a recommendation for excision of the area due the underlying distortion. On the other hand, surgeons may prefer to know before surgery if the pathologic finding is consistent with radial scar, requiring only a small volume excision, or invasive carcinoma, which requires a larger volume excision and nodal sampling. Hence, core biopsy can aid in biopsy planning ( Box 11-3 ).
- •
Let the patient know that the lesion may be benign but will need to be surgically removed because of the risk of associated carcinoma
- •
Arrange appointments for core biopsy and surgical consultation
- •
Perform core needle biopsy for surgical planning:
- •
If the lesion is benign, perform wire localized surgical excision
- •
If it is carcinoma, evaluate for extent of disease and nodal metastasis. Perform wire localized lumpectomy if breast conserving therapy is desired.
- •
If a core biopsy is performed for a different type of BI-RADS 4 lesion, such as calcifications or a focal asymmetry, and shows an incidental minute radial scar that was completely contained within the core sample, excisional biopsy is probably not necessary.
Subtypes of IDC
IDC-NOS is the most common type of breast cancer. Essentially, the carcinoma is differentiated enough to form ducts, but that is all. There are several subtypes of IDC that are better differentiated—they make mucin, form tubules, etc. Because these cancers are more differentiated, they tend to grow slowly (except medullary carcinoma) and be circumscribed (except tubular). They are typically grade I and have a better prognosis than IDC-NOS overall ( Boxes 11-4 and 11-5 ).
- •
Tubular
- •
Medullary
- •
Mucinous
- •
Papillary
- •
IDC-NOS
- •
Medullary
- •
Mucinous
- •
Papillary
- •
Phyllodes
Tubular carcinoma is characterized by the formation of tubules or small ductules. It typically presents as a small mass, often with long spicules ( Fig. 11-5 ) like cat whiskers, and is often multifocal. Tubular carcinoma has an excellent outcome with a cause-specific survival rate of 98% at 10 years. Axillary metastasis is very uncommon, and distant disease is even less common. If you could pick your type of invasive cancer to be diagnosed with, this is the one.
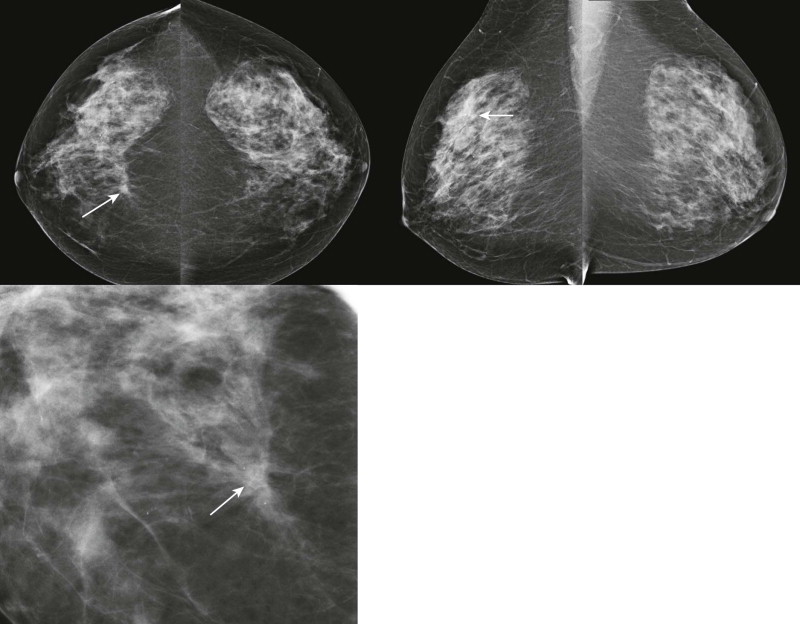
One of our prior fellows (a “Valley Girl” from California), pointed out that “tubular” was synonymous with “cool” during the 1980s. So think, “Like, tubular, man!” and you’ll remember that this is the cool, easy-going invasive cancer despite the appearance of a spiculated mass. A word of caution—if you have teenage kids, we don’t recommend using this language (or related terms like “nifty”) in their presence unless you really want to see their eyes roll!
Histologically, tubular carcinoma can mimic a radial scar and vice versa. The pathologist may use actin stain to check for myoepithelial cells that are present in the basement membrane of tubules associated with radial scar, but absent in tubular carcinoma.
Medullary carcinoma is a very rapidly growing cancer that typically presents as a palpable mass in young or middle-aged women ( Fig. 11-6 ). The median age of diagnosis is 51. “Medulla” means marrow. Like basal cell (triple negative) IDC, medullary carcinoma is characterized by a brisk lymphocytic response, absence of fibroglandular differentiation, and pleomorphic nuclei. There is usually little or no associated DCIS. So at first glance under the microscope medullary carcinoma resembles an aggressive IDC. There is considerable interobserver variability in the pathologic diagnosis of medullary carcinoma that may explain the variable patient outcomes reported across different series. The prognosis for women with this cancer is better than for typical IDC in most series.
On mammography, medullary carcinoma typically presents as a round or oval, circumscribed mass without calcifications. It is often palpable.
Mucinous (colloid) carcinoma is characterized by tumor cells floating in a pool of mucin ( Fig. 11-7 ). This lesion presents most commonly in older women with a median age of 71. Your last experience with an upper respiratory infection should remind you that mucin is a soft semiliquid. As a result, the mammographic appearance is usually a low-density mass rather than a dense clump of cells like most cancers ( Fig. 11-8 ). The margins are typically fairly well-circumscribed. The US appearance can be relatively isoechoic, and often there is posterior acoustic enhancement. Like proliferative fibroadenomas, these masses are very T2 hyperintense on MRI, but tend to be more heterogeneous on T1 postcontrast sequences.