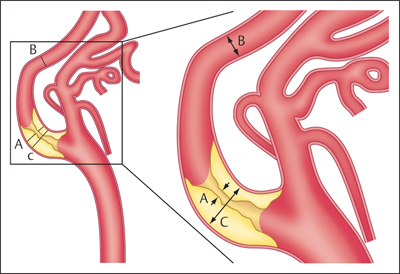
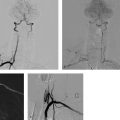
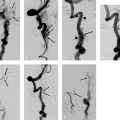
11 Extracranial Carotid Stenoses The interventional treatment of atherosclerotic stenoses of the common carotid artery (CCA) and internal carotid artery (ICA) began in the late 1970s and early 1980s, consisting initially of conventional percutaneous trans-luminal balloon angioplasty. One of the first reports on this treatment was published by Kerber et al. (1980). The number of publications on this subject grew steadily thereafter, with most authors reporting good clinical outcomes (Bockenheimer and Mathias 1983; Tievsky et al. 1983; Wiggli and Gratzl 1983; Tsai et al. 1986). A high rate of restenosis was a serious initial problem, however (Diethrich et al. 1995). Carotid artery stenting was first performed in 1989 to reappose an intimal flap caused by percutaneous transluminal balloon angioplasty. The first report of a carotid stenosis treated by stent angioplasty was published in 1995. It involved the treatment of a recurrent stenosis following thromboendarterectomy (Mathias et al. 2001). When stent angioplasty was found to decrease restenosis rates and yield better long-term results, it began to replace conventional percutaneous transluminal balloon angioplasty in the interventional treatment of carotid stenosis. The results of the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) I trial (CAVATAS Investigators 2001) in particular were instrumental in showing the superiority of stent-assisted percutaneous transluminal balloon angioplasty over conventional percutaneous transluminal balloon angioplasty. Since then, stent-assisted percutaneous transluminal balloon angioplasty has become the endovascular procedure of first choice for carotid stenosis. Standard techniques have been devised, and researchers have developed a range of new self-expanding stents designed for specific types of stenosis. Embolic protection systems were introduced in the late 1990s in the belief that they could reduce the risk of peri-interventional embolization. A variety of systems have been developed over the years, with distal filter systems becoming the most popular. Various publications in subsequent years sought to document the protective effect of these systems over unprotected stenting (Reimers et al. 2001; Castriota et al. 2002; Cremonesi et al. 2003). The interventional treatment of carotid stenosis requires only a small inguinal stab incision, as opposed to a longer cervical incision. The latter carries a risk of lower cranial nerve injury and creates a larger wound area. This may be particularly objectionable in female patients and is often cited as a reason for favoring an interventional technique. The procedure times for interventional and surgical treatment are comparable (~1 h). Interventions are generally performed under local anesthesia, whereas surgical treatment usually requires general endotracheal anesthesia. One problem in previous randomized studies comparing surgical carotid endarterectomy with interventional carotid artery stenting has been the demonstrably higher incidence of minor strokes associated with interventional treatment. There is considerable evidence, however, that proper stent selection, sound interventional technique, and adequate operator experience can reduce the complication rate of carotid stenting to a level that makes it competitive with surgery. There is still considerable debate as to the value of using protection systems in interventional procedures, but the analysis of data from large randomized prospective trials (SPACE I, EVA 3S, and ICSS; see below) indicates advantages for unprotected stenting. The results of the ECST (European Carotid Surgery Trial; European Carotid Surgery Trialists’ Collaborative Group 1998) and NASCET (North American Carotid Endarterectomy Trial; Barnett et al. 1998) trials have shown that surgical treatment can significantly reduce the risk of stroke in patients with a symptomatic high-grade carotid artery stenosis (NASCET: > 50%). A subanalysis of NASCET data on asymptomatic contralateral ICA stenoses in patients symptomatic for the other side showed that the risk of stroke from the asymptomatic stenosis was significantly reduced only in cases where the degree of stenosis (NASCET) was less than 60% (Inzitari et al. 2000). The ECST trial studied a total of 3024 patients at 97 centers from 12 European countries and one Australian center during the period 1981–1994. Of that total, 1811 patients were randomly assigned to the surgical arm and 1213 to the control arm, in which surgical treatment was avoided for as long as possible. The study found that the Kaplan-Meier estimate of the frequency of a major stroke or death at 3 years was 26.5% in the control arm vs. 14.5% in the surgical arm in patients with > 80% carotid stenosis (ECST). The authors conclude that surgical treatment is indicated for patients with symptomatic carotid stenosis > 80% (ECST). This corresponds to a ~65% stenosis by the NASCET criteria. The NASCET trial, conducted at 106 American centers from 1987 to 1996, randomly assigned 2226 patients with symptomatic carotid stenosis to a medical arm (1118 patients) and a surgical arm (1108 patients). Two groups were formed based on the degree of initial stenosis: one group with < 50% stenosis (NASCET) and a second group with 50–69% stenosis (NASCET). After 5 years, the rate of ipsilateral stroke in the group with 50–69% stenosis (NASCET) was 15.7% in patients treated surgically vs. 22.7% in patients treated medically. In the group with < 50% stenosis (NASCET), no statistically significant difference was found at 5 years between the surgically and medically treated patients (Barnett et al. 1998; European Carotid Surgery Trialists’ Collaborative Group 1998). Note Based on these study findings, treatment is currently recommended for patients with symptomatic carotid stenosis > 50% (NASCET), but only if the complication rate associated with the treatment is < 6% at the institution in question. Thus far, data on degree of stenosis have been based on the NASCET method of measurement, in which the residual lumen within the stenosis is measured in relation to the closest unaffected distal vascular segment with parallel walls. This method is employed in digital subtraction angiography (DSA), CT angiography (CTA), and MR angiography (MRA). By contrast, the ECST method is based on a Doppler ultrasound technique in which the original diameter of the vessel lumen is determined at the affected site and the stenosis is measured relative to the original lumen. This cannot be done in the NASCET method, which is based on angiographic measurements (Fig. 11.1.) Fig. 11.1 The ECST and NASCET methods for determining degree of stenosis. (A/C) × 100 = degree of stenosis (%) by the ECST method (A/B) × 100 = degree of stenosis (%) by the NASCET method The following formula can be used to convert between the measurements: ECST stenosis (%) = 0.6 × NASCET stenosis (%) + 40% Patients with asymptomatic carotid stenoses have a significantly lower risk of stroke. Even so, it may generally be assumed that the age-independent 5-year risk is < 10% (Halliday et al. 2004), and that the stroke risk for women is almost 2.5% lower than that for men. The ACST (Asymptomatic Carotid Surgery Trial) trial compared surgical and medical treatment in patients with asymptomatic carotid stenosis and found that surgical treatment provided a significant benefit in terms of risk reduction only if the complication rate of the treatment was < 3%. This seems reasonable until we consider that the corresponding number needed to treat to prevent one stroke is 53! Wholey et al. (2003) published the results of a registry (Multi-Centre World Carotid Registry) reviewing the results of carotid stenting regardless of the exact procedure used (including the use of protection devices). In a total of 6734 stent placements, they found a 30-day complication rate of 5.8% for ipsilateral strokes. Garg et al. (2009) took a somewhat more differentiated approach in a review of 134 publications from 1995 to 2007 that met the authors’ inclusion criteria. A pooled analysis of these 134 publications showed that carotid stenting was associated with a relative risk of 0.62 (95% confidence interval [CI] 0.54–0.72) for ipsilateral stroke. A subgroup analysis revealed a significant benefit from protected stenting in symptomatic patients (relative risk [RR] 0.67; 95% CI 0.52–0.56) and asymptomatic patients (RR 0.61; 95% CI 0.41–0.90; p < 0.05). In the late 1990s, various prospective randomized multicenter studies were planned to compare stent angioplasty of the carotid artery with conventional carotid endarterectomy. The EVA-3S trial (Endarterectomy vs. Angioplasty in Patients with Symptomatic Severe Carotid Stenosis), which began in 2000 and was conducted at 30 centers in France, was stopped early in 2005 after the enrollment of 527 symptomatic patients for ”reasons of both safety and futility” in showing equality between interventional and surgical treatment. The initial degree of stenosis necessary for enrollment was 60–99% (NASCET). The complication rate for stroke and death was 3.9% (95% CI 2.0–7.2) after carotid endarterectomy and 9.6% (95% CI 6.4–14.0) after carotid stent angioplasty (Mas et al. 2006). SPACE I (Stent-Protected Percutaneous Angioplasty of the Carotid Artery vs. Endarterectomy) was a more rigorously designed and conducted trial involving 35 centers in Germany, Austria, and Switzerland. The results published in 2006 could not demonstrate noninferiority of stent angioplasty compared with surgical treatment. Between 2001 and 2006, a total of 1200 symptomatic patients with an initial stenosis of at least 50% (NASCET) or 70% (ECST) were randomly assigned to an interventional arm (605 patients) and a surgical arm (595 patients). The final analysis encompassed 599 patients in the stent arm and 584 patients in the carotid endarterectomy arm. The primary endpoint of ipsilateral stroke or death was reached in 6.84% of the patients with carotid artery stenting and 6.34% of the patients with carotid endarterectomy (Ringleb et al. 2006). The ICSS (International Carotid Stenting Study) did not yield a positive result (Ederle et al. 2010). The trial enrolled 1713 patients at 50 European, Australian, New Zealand, and Canadian academic centers between 2001 and 2008. Seven hundred fifty-one (88%) of 853 patients in the stent arm and 760 (89%) of 857 patients in the surgical arm were finally treated and evaluated. The incidence of stroke, death, or myocardial infarction between randomization and 120 days was 8.5% after carotid stent angioplasty and 5.2% after carotid endarterectomy (72 vs. 44 events; hazard ratio = 1.69; 95% CI 1.16–2.45; p= 0.006; Ederle et al. 2010). Note Previous subanalyses of randomized prospective studies show benefits for unprotected stenting with regard to peri-procedural complication rate, and the ICSS MRI subgroup exhibited fewer postinterventional DWI lesions with unprotected stenting (Jansen et al. 2009; Bonati et al. 2010). Although the differences between the procedures related chiefly to minor strokes in all the studies, it was obvious that stent angioplasty was increasingly called into question as a treatment for carotid artery stenosis. For this reason, the results of the CREST study (Carotid Revascularization Endarterectomy vs. Stenting Trial), still in progress at that time, were awaited with great interest. The CREST study enrolled 2502 symptomatic and asymptomatic patients at 108 centers in the U.S. and Canada from 2000 to 2008. The initial degree of stenosis had to be > 50% (NASCET) or > 70% (ECST) at time of enrollment. The 4-year rate of stroke or death was 6.4% with stenting and 4.7% with endarterectomy. When the patients were divided into symptomatic and asymptomatic groups, the respective rates were 8.0 vs. 6.4% and 4.5 vs. 2.7% in the stent and surgical arms. The rates for specific periprocedural end points were as follows: 0.7 vs. 0.3% for death, 4.1 vs. 2.3% for stroke, and 1.1 vs. 2.3% for myocardial infarction in the interventional and surgical arms. Thus, while carotid stenting was associated with a slightly higher risk of strokes (mostly minor), more myocardial infarctions were observed in the surgical arm. Overall, the authors of the CREST study concluded that the composite risk profiles were comparable in the groups undergoing carotid stenting and carotid endarterectomy (Brott et al. 2010). Previous study data also indicate a tendency for younger patients to benefit more from stenting while older patients benefit more from surgery. Meier et al. (2010) performed a meta-analysis of the results from 11 studies comparing carotid endarterectomy and carotid artery stenting. For the primary composite endpoint of the risk of periprocedural stroke or death, the meta-analysis shows a significantly better result for carotid endarterectomy than carotid stent angioplasty (OR 0.67; 95% CI 0.47–0.95). This result related chiefly to a lower risk of strokes with carotid endarterectomy than with carotid artery stenting (OR 0.65; 95% CI 0.43–1.00; p= 0.049). The difference was driven mainly by the occurrence of minor (nondisabling) strokes. As in the CREST trial, surgical treatment was associated with a significantly higher risk of myocardial infarction than interventional treatment (OR 2.69; 95% CI 1.06–6.79; p= 0.036). Moreover, the data showed for the first time that the risk of lower cranial nerve injury or cervical nerve injury was significantly higher after carotid endarterectomy. The confidence intervals of the results are very broad, however. This highlights the heterogeneity of the results from different studies and the small case numbers, which do not provide a basis for drawing reliable conclusions (Meier et al. 2010). Note In summary, the results to date show that even the large, multinational multicenter studies have not provided adequate case numbers in order to draw evidence-based conclusions for or against the interventional or operative treatment of carotid stenosis. Despite the lack of definitive statistical evidence, there are initial situations that would definitely favor carotid artery stenting over carotid endarterectomy. For example, stenting is preferable in cases where a stenosed ICA is poorly collateralized by a deficient circle of Willis. This type of case could also be described as an ”isolated” carotid artery. Moreover, carotid endarterectomy would be difficult or impossible to perform in patients with a high carotid bifurcation located at a poorly accessible site behind the mandibular angle. The same applies to other surgically inaccessible stenoses of the proximal CCA and distal ICA. Also, tandem stenoses consisting of a bifurcation stenosis plus a stenosis of the proximal CCA or distal ICA would not be surgically treatable in most cases. Recurrent stenosis after carotid endarterectomy, radiogenic stenosis, and carotid stenosis following a neck dissection (generally combined with radiation) would also contraindicate surgery. An occlusion of the contralateral ICA or a high-grade stenosis is associated with a significant increase in perioperative morbidity (see also NASCET study, earlier). Another important consideration is comorbid conditions, especially preexisting cardiac and cardiovascular diseases, which would tend to favor carotid artery stenting because of the increased risks involved in general anesthesia. In principle, carotid stent angioplasty can be performed with or without the use of protection systems. Embolic protection systems are available in various designs. The most widely used devices are net-type filters, and the most popular device of this kind is the FilterWire EX (Boston Scientific, Natick, MA, USA). It is a small filter (< 3.5F) mounted on a 0.014-in (0.36-mm) microwire. With a pore size of 80 μm, the filter permits antegrade blood flow while preventing the distal embolization of debris. The design is characterized by an eccentrically placed filter with a ”fish-mouth” opening. A radiopaque nitinol framework provides for stability and adequate visibility on radiographs. Other protection systems based on the distal filter concept are AccuNet, AngioGuard, Emboshield, Interceptor PLUS, NeuroShield, SPIDER, and Rubicon. Another concept is the balloon occlusion system, which may be of the distal or proximal occlusion type. Proximal balloon occlusion systems are further subdivided into devices that interrupt flow and devices that produce retrograde flow via an inguinal arteriovenous shunt. A proximal balloon occlusion system should be considered only for a very high-grade ulcerating stenosis with a high-risk MRI profile or pronounced kinking just past the stenosis to avoid unprotected crossing of the stenosis. Examples of proximal balloon protection systems are the Parodi and MO.MA systems and the Kachel balloon. Distal balloon protection systems are illustrated by the PercuSurge, Guardwire Plus, and Tri Activ System. Note Basic disadvantages of distal protection systems are the unprotected crossing of the stenosis with the protection device as well as incomplete protection against small emboli, which has been demonstrated in various studies. Proximal systems avoid these problems but require large-gauge inguinal access (up to 10F). The available carotid stents can be classified as having either an open- or closed-cell design. In a closed design, all the cells in the stent are interconnected with one another to form a structure similar to a knitted stocking. In an open design, there are gaps in the latticework that give the stent greater flexibility. One drawback of the open-cell design is that the omission of struts may increase the risk of embolization during postdilatation, especially in elongated vessels. By contrast, some closed stent designs have extremely small cells that offer better embolic protection by fixing the plaque against the vessel wall, but these stents have a tendency to straighten an elongated vessel and may cause kinking of the vessel at the distal end of the device. The best-known stent for the treatment of atherosclerotic carotid stenosis is the Carotid Wallstent (Boston Scientific Corp., Natick, MA, USA). It has a closed-cell design composed of braided wires made of a cobalt-chromium-iron-nickel-molybdenum alloy (commonly known as Elgiloy or Conichrome) and has the smallest pore size available on the market (1.08 mm2). Other frequently used carotid artery stents are listed below: • Acculink (Guidant Corporation, Santa Clara, CA): laser-cut nitinol stent with open-cell design. • OptiMed Sinus-Carotid (Conical) RX (OptiMed, Ettlingen, Germany): nickel-titanium alloy stent with open-cell design. One model has a tapered shape conforming to the caliber change at the CCA-ICA junction. • Precise (Cordis Endovascular, Warren, NJ, USA). • Cristallo ideale (Invatec, Roncadelle, Italy): nitinol hybrid stent with a closed-cell design in the central part to prevent embolism and an open-cell design in the distal and proximal sections for better conformability. • NexStent (Boston Scientific Corp., Natick, MA, USA): This stent does not have a closed tubular shape like other stents but consists of a rolled nitinol sheet with a closed-cell design and a pore size of ~4 mm2. It is claimed that this rolled design enables the stent to conform to a range of vessel diameters (4–9 mm). Practical Tip When placing stents with an open-cell design, it may be advantageous to use a protection system. A closed-cell stent with a small cell size will generally provide adequate embolic protection and can be safely used without an extra protection system (Jansen et al. 2009).
Introduction
Indications
Study Results
Clear Indications for Stenting
Materials
Protection Systems
Carotid Stents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree