Eye
Evangelos S. Gragoudas
John E. Munzenrider
Anne Marie Lane
Michael J. Collier
Radiation therapy was first used successfully for the treatment of intraocular tumors in 19291 and by the 1960s, Stallard was using cobalt-60 (60Co) plaques to treat choroidal melanomas.2,3 However, the morbidity from the deeply penetrating 1.17 to 1.33 MeV gamma irradiation associated with cobalt brachytherapy was quite high. Therefore, alternative brachytherapy plaque sources as well as external beam approaches including heavy charged particles were evaluated. Currently, two radiotherapy techniques are predominantly used—heavy particle radiation and plaque brachytherapy.
Brachytherapy uses radioactive plaques sutured on the sclera over the tumor.4, 5, 6 External beam irradiation includes the use of charged particles such as protons7 and helium ions.8 Despite good results achieved with helium ion irradiation,9 it is no longer used due to its high cost. More recently, radiosurgery with the Gamma Knife10,11 has been used to treat choroidal melanomas, but the dose distributions are not as attractive as those achieved with proton irradiation,10 and long-term follow-up data are not yet available for efficacy analyses.
Controversy continues to exist as to which of these radiotherapeutic modalities is the most appropriate to treat choroidal melanoma. The metastatic rates appear to be similar among the various types of radiotherapy. Visual outcome and local recurrence may then become the deciding factor in choosing the type of treatment. Treatment with heavy particles such as protons and helium ions has reduced local recurrences12,13 and may improve visual prognosis because the properties of charged particles are such that close to 100% of the energy is delivered to the target tissue, with very little exposure of surrounding healthy tissue. The lower radiation doses to normal tissues result in less radiation damage to healthy structures such as the macula or optic disc, potentially allowing visual acuity to be maintained.
RATIONALE FOR PROTON RADIOTHERAPY
The advantages of charged particle irradiation are based on the physical characteristics of protons and helium ions that make possible highly localized and uniform radiation dose distributions. The uniform dose of radiation delivered to the whole tumor and the sharp reduction of the dose outside the treated area increases the proportion of large ocular tumors that can be treated because the overall irradiated volume is reduced, thereby improving the therapeutic ratio of local control versus complications. Likewise, tumors close to critical structures (macula, optic nerve) can be irradiated and the eye can still maintain visual potential. Histologic examination of irradiated tumors provides evidence of these advantageous properties of protons. In one study, vessel thrombosis occurred exclusively in retina overlying the tumor,14 and in another, the choroidal vasculature and retinal pigment epithelium (RPE) were reduced or absent beneath and above the melanoma but were intact adjacent to it.15
PATIENT SELECTION
Patients with large uveal melanomas (up to 24 mm in diameter and 14 mmin height) may be treated with charged particle irradiation. In general, large tumors that are located at the peripheral fundus have a better prognosis in terms of visual function than large tumors near critical structures because the beam can enter the eye directly in the area of the tumor and does not need to traverse noninvolved ocular structures. Tumors involving the macula and/or optic disc and small extrascleral extensions can be treated. Our experience indicates that the eye can tolerate irradiation of up to 30% of its volume with the currently used doses. For
tumors involving >30% of the ocular volume, enucleation is recommended as it is for tumors with large extrascleral extension or extensive iris neovascularization. Lesions <10 mm in diameter and 2 mm in height are usually not treated until tumor growth has been documented.
tumors involving >30% of the ocular volume, enucleation is recommended as it is for tumors with large extrascleral extension or extensive iris neovascularization. Lesions <10 mm in diameter and 2 mm in height are usually not treated until tumor growth has been documented.
DIAGNOSTIC EVALUATION, STAGING, AND TARGET DEFINITION
The diagnosis is established through an ophthalmologic examination and ancillary tests, fluorescein angiography and ultrasonography, which is used also to determine the height of the tumor. All patients also undergo a systemic examination by an internist, with chest radiography and liver function studies, to exclude metastasis or other primary malignancy. If the examination or the laboratory tests show an abnormality, ultrasonography, computed tomography, or magnetic resonance imaging (MRI) of the liver/abdomen is performed. If these tests are negative, and the decision to proceed with radiation treatment is made, the tumor is localized surgically16,17 in preparation for radiotherapy.
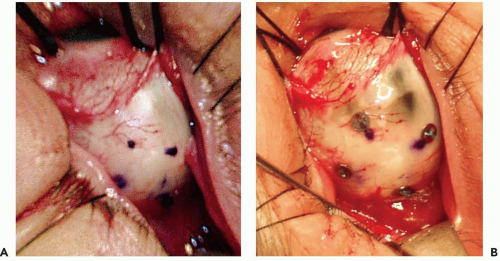
The conjunctiva is incised and the tumor is localized by transillumination and/or indirect ophthalmoscopy. The edges of the tumor are marked with a blue pencil (see Fig. 13.1a), and four tantalum rings 2.5 mm in diameter are sutured to the sclera to outline the tumor (see Fig. 13.1b). Ring to limbus distances (ring to anterior margin distances for tumors that extend into the ciliary body and iris) and distances between the rings are measured. The largest tumor diameter is measured with calipers on the sclera after the tumor margins have been defined. The extrascleral tissues over the area of the tumor are examined carefully for evidence of extrascleral extension. Very posterior tumors, especially tumors abutting the optic nerve, have rings placed only at the anterior and lateral margins of the tumor, and the distance from the rings to the posterior margin is estimated from fundus photographs. The tumor is transilluminated again after suturing of the rings, the distances of the ring from the tumor base are measured, and careful drawings of the shape of the tumor in relation to the rings are made. In tumors confined to the ciliary body and iris,18 no surgical localization is required. The margins of the tumor are identified by transillumination, and defined in relation to anatomic landmarks of the iris and conjunctiva on the ocular surface.
PROTON RADIOTHERAPY TREATMENT
Treatment Planning
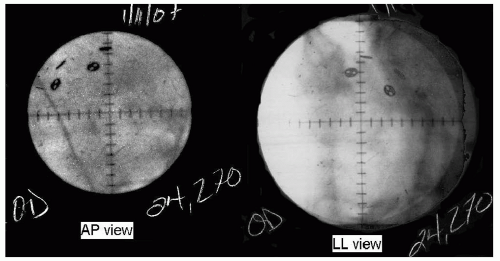
After the surgery, the planning19 of the treatment begins with orthogonal pairs of x-rays taken of the eye with the patient gazing in three different directions (see Fig. 13.2). For each gaze direction, the positions of the four rings are measured in three dimensions. Then, using this information and the ultrasonorgaphic measurement of the axial length of the eye, the planning softwarea creates a spherical model of the globe chi-square fitted to those four points in space. From the knowledge of the gaze direction, the software adds the structures of the eye to the sphere; the depth and thickness of the lens from ultrasonographic
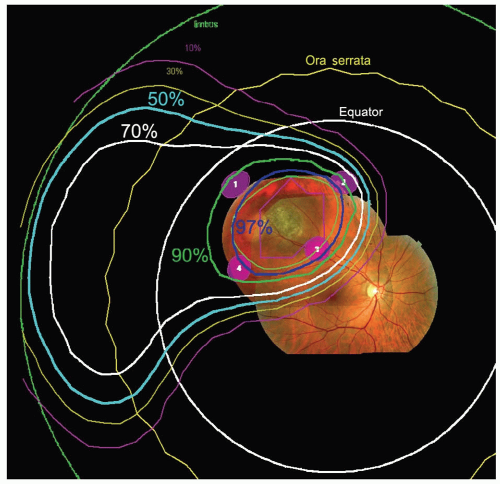
measurements are entered. Next, the base of the tumor is drawn relative to rings as observed during the surgery. This can be done on wide angle (see Fig. 13.3) and ringcentered views of the fundus, or on an anteroposterior (AP) view of the iris for iris tumors. Also photographs of the fundus (or iris) can be projected on a wide-angle fundus view (Fig. 13.3) (or anterior iris view) to aid in drawing the base in any region that is not near a ring. The fundus photographs are especially useful for very posterior tumors and tumors abutting the optic nerve where it is impossible to surround the tumor with marker rings. The tumor height is also entered and an apical surface created, usually parabolic in shape, to complete the three-dimensional model of the tumor. The program allows two tumors to be created; this feature is needed if there is extrascleral extension, a ciliary-body tumor that also invades the iris, or a tumor that has a more irregular shape. Tumors in the iris or ciliary body, for which placement of marker rings is unnecessary, are drawn from clinical and ultrasonographic information.
measurements are entered. Next, the base of the tumor is drawn relative to rings as observed during the surgery. This can be done on wide angle (see Fig. 13.3) and ringcentered views of the fundus, or on an anteroposterior (AP) view of the iris for iris tumors. Also photographs of the fundus (or iris) can be projected on a wide-angle fundus view (Fig. 13.3) (or anterior iris view) to aid in drawing the base in any region that is not near a ring. The fundus photographs are especially useful for very posterior tumors and tumors abutting the optic nerve where it is impossible to surround the tumor with marker rings. The tumor height is also entered and an apical surface created, usually parabolic in shape, to complete the three-dimensional model of the tumor. The program allows two tumors to be created; this feature is needed if there is extrascleral extension, a ciliary-body tumor that also invades the iris, or a tumor that has a more irregular shape. Tumors in the iris or ciliary body, for which placement of marker rings is unnecessary, are drawn from clinical and ultrasonographic information.
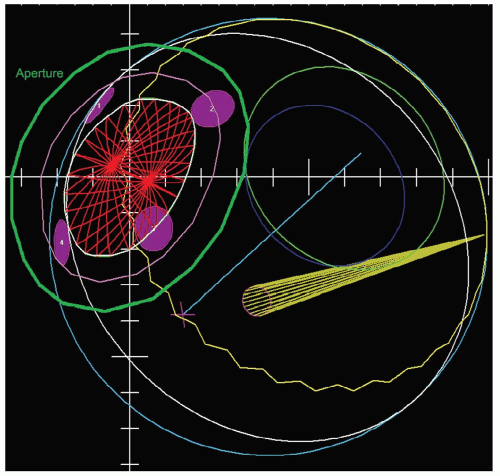
The gaze direction of the eye for treatment is chosen to give the maximum separation possible between the tumor and the critical structures (lens, cornea, macula, optic disc) either in the beam’s-eye view (see Fig. 13.4) or in-depth to give minimum dose to these structures. The program automatically designs an aperture that gives a margin around the tumor usually chosen to be 3 mm (1.5 mm for the 90% to 50% lateral penumbra and 1.5 mm for uncertainties in tumor border and daily set up). This margin may be reduced to 2.5 mm for a tumor border at the limbus or increased to 4 mm for a patient without surgical markers. The program calculates the maximum and minimum depths of the tumor and allows the user to choose proximal and distal margins to give the needed beam range and modulation. The standard distal margins are 4 mm for posterior tumors because there is only retroorbital fat distal to the tumor, 3 mm for equatorial tumors, and 2.5 mm for anterior tumors because the macula and disc are
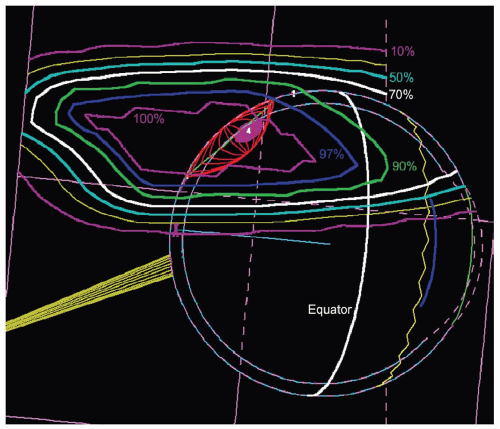
frequently directly distal to the tumor. The program calculates dose distributions on the fundus displayed in the geometry of a wide-angle fundus photograph (Fig. 13.3) and in any plane through the eye (see Fig. 13.5). It also calculates dose-volume histograms for the tumor and many structures of the eye (see Fig. 13.6).
frequently directly distal to the tumor. The program calculates dose distributions on the fundus displayed in the geometry of a wide-angle fundus photograph (Fig. 13.3) and in any plane through the eye (see Fig. 13.5). It also calculates dose-volume histograms for the tumor and many structures of the eye (see Fig. 13.6).
Patient Immobilization
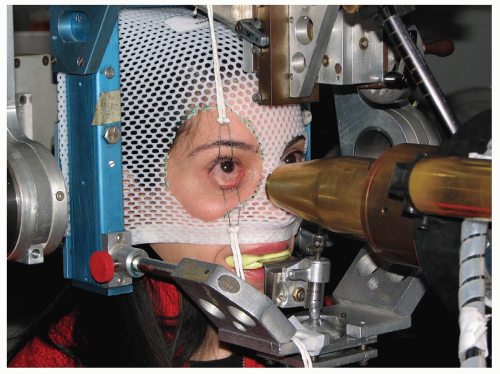
A high degree of accuracy in patient positioning is achieved with a headholder attached to the proton beam collimator (see Fig. 13.7). The headholder allows controlled rotation of the head around two mutually perpendicular axes intersecting at a point that is positioned accurately on the axis of the collimator. The patient is seated in a specially designed chair and his head is immobilized with a bite block made of dental impression compound and a modified face mask fastened to the headholder. Orientation of the patient’s eye is established by voluntary fixation of the eye being treated (the other is covered) on a small light attached to the collimator. If vision is poor in the eye being treated, the other eye can be used for fixation. The eyelids are held open with a lid speculum, placed after instillation of topical anesthetic drops into the eye.
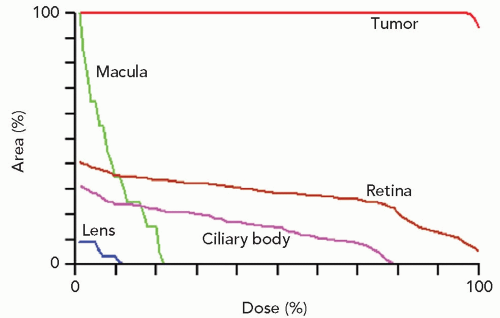 Figure 13.6 Dose-volume (or area) histograms for the tumor and four of the more critical structures of the eye (the optic disc received no discernible dose). |
Image Guidance and Target Verification
Each patient port is calibrated to determine the true depths of the proximal and distal 90% doses (which must be within 1 mm of the prescribed depths) and the number of monitor units needed to deliver the prescribed dose in the flat spread-out Bragg peak. The calibration is performed with a polymethyl methacrylate (PMMA) phantom and a silicon diode detector because its small size allows it to be used for even the smallest apertures, <1 cm in diameter. The diode itself is calibrated against a T1 thimble ionization chamber.20
The eye is viewed with a high-magnification closed-circuit television system with its effective viewing point on the beam axis. This system provides a magnified image 10 times the size of the patient’s eye and permits continuous monitoring of the eye’s position throughout the procedure. The alignment of the particle beam is achieved with orthogonal x-rays. The tumor, defined spatially by the radiopaque tantalum rings, can be positioned by translating the headholder until it is in the desired
position relative to the beam axis. Positioning is achieved with a fluoroscopic system that provides a virtually instantaneous picture held on an image storage device. This system hastens alignment and assists in confirming eye immobilization during treatment. The alignment procedure lasts approximately 15 minutes. The patient is asked to fixate, and the eye’s position is observed on a television monitor in the control area. The treatment field is checked with a beam simulation field light, and if the position and fixation are satisfactory, the treatment begins. If eye movement of >0.5 mm is observed, the treatment is halted immediately. Most treatments, however, are delivered uninterrupted in 1 to 2 minutes. Mean movement during treatment of 0.5 ± 0.3 mm was observed in a group of treated patients with maximal movement of 1.2 mm.21 This degree of accuracy of voluntary fixation has convinced us that paralytic fixation or electronic interlock is unnecessary.
position relative to the beam axis. Positioning is achieved with a fluoroscopic system that provides a virtually instantaneous picture held on an image storage device. This system hastens alignment and assists in confirming eye immobilization during treatment. The alignment procedure lasts approximately 15 minutes. The patient is asked to fixate, and the eye’s position is observed on a television monitor in the control area. The treatment field is checked with a beam simulation field light, and if the position and fixation are satisfactory, the treatment begins. If eye movement of >0.5 mm is observed, the treatment is halted immediately. Most treatments, however, are delivered uninterrupted in 1 to 2 minutes. Mean movement during treatment of 0.5 ± 0.3 mm was observed in a group of treated patients with maximal movement of 1.2 mm.21 This degree of accuracy of voluntary fixation has convinced us that paralytic fixation or electronic interlock is unnecessary.
During treatment, the proton beam passes through a variable thickness piece of Lexan22 which serves as single scatterer to spread the beam laterally and degrade the beam energy to match the prescribed depth in the eye. A modulator wheel, made of plastic with steps of different thicknesses, spins in the proton beam to create the appropriate spread-out Bragg Peak to encompass the tumor plus margins.23 For a specific treatment, the appropriate modulator is chosen from a library of wheels or a new one is milled out of plastic (PMMA).
Fractionation
At our facility, the standard treatment regimen is a dose of 70 Gy (RBE) delivered in five equal fractions in 5 days (RBE = 1.1). A somewhat lower dose of 60 Gy (RBE) in 4 equal fractions over 4 days is favored at several proton facilities in Europe.12,24, 25, 26 We selected large fractions based on favorable clinical results with a small number of relatively large fractions27,28 for patients with skin melanomas. At the prescribed dose of 70 Gy (RBE), we estimate that the optic nerve and macula receive the full dose (70 Gy [RBE]) when the tumor is <1 mm from these visual structures, approximately 35 Gy (RBE) when the tumor is located 3 mm from these structures, and a small to negligible dose of ≤15 Gy (RBE) when the tumor is peripheral (beyond 9 mm). Although most of our patients have been treated with 70 Gy (RBE), we have evaluated other treatment regimens including a small number of tumors treated early in the program at doses up to 100 Gy (RBE) and 94 patients who received 50 Gy (RBE) as part of a randomized clinical trial to establish safety and efficacy of dose reduction.29 We found no significant differences in tumor control and ocular complications between patients who received the standard dose and those who received the lower dose. Therefore, the optimal fractionation scheme and dose level to achieve tumor control with minimal ocular morbidity remains to be determined.
CLINICAL RESULTS
The first patient to be treated with protons for uveal melanoma was treated at the Harvard Cyclotron in 197530and at present >3,000 patients with uveal melanomas have been treated through a collaboration of the Massachusetts Eye and Ear Infirmary (MEEI)/Massachusetts General Hospital (MGH).13 Lifetime follow-up is completed for all treated patients who agree to enroll in a uveal melanoma registry, and data obtained through these follow-up efforts have been used to evaluate the efficacy and safety of proton therapy in terms of visual and survival endpoints.
As is the case with many cancers, diagnosis before age 30 is unusual (3% of patients were diagnosed before their 30th birthdays, 9% of patients were diagnosed between 30 and 39, and 14% of patients were diagnosed between 40 and 49 in the Harvard cohort). Bilateral involvement and extrascleral extension are also uncommon, with each occurring in <5% of patients. Less than 10% of tumors originated in the ciliary body as opposed to the choroid (i.e., >75% of the AP diameter fell anterior to the ora serrata, the posterior delimiter of the ciliary body). The average largest basal diameter of the tumor was 13.2 mm (range: 5.0 mm to 28.0 mm); the average height was 5.3 mm (range: 0.6 mm to 17.1 mm); most tumors (63%) covered 10% or less of the total uveal tract (based on the area of the tumor’s largest diameter and perpendicular diameter), whereas a small number of tumors (5%) involved 25% or more of the uveal surface. Approximately 26% of patients had tumors classified as T1 (no more than 10 mm in diameter and 2.5 mm in height), and approximately half had T2 tumors (>10 mm but no more than 16 mm in diameter and/or >2.5 mm but no more than 10 mm in height). Approximately 20% had large tumors (>16 mm in diameter and/or > 10 mm in height) but only 2% were diagnosed with T4 tumors, (large tumor with extrascleral extension). Most patients had some deterioration in visual acuity at presentation, but very few experienced severe vision loss (counting fingers or worse) at the time of diagnosis.
Local Control
The hallmark of local control is no increase in size of the tumor. Most tumors show a decrease in size, which continues over years. Tumor regression is due mainly to irradiation-induced destruction of tumor cells (through chromosomal DNA injury), but damage to the tumor vasculature may also contribute to regression. Histopathologically, irradiation effects include patchy areas of necrosis, proteinaceous exudates, lipid vacuoles in the cytoplasm, pyknotic nuclei, increased number of filaments, breakdown of mitochondria, and balloon cell formation.15 Significantly fewer mitotic figures in forty high-power fields were observed in irradiated tumors that were subsequently enucleated compared to enucleated eyes that did not receive
prior radiation treatment.14 Vascular changes include endothelial cell swelling, decreased lumen size, basement membrane thickening, collapse of sinusoidal vessels, and vessel thrombosis. The typical course of regression begins a few months following irradiation, and tumors may continue to regress years after treatment.31 Complete disappearance of the tumor is unusual (see Fig. 13.8). This protracted pattern of tumor regression has been supported by histologic studies among controlled tumors that were enucleated at varying times after proton therapy; although mitotic figures declined with longer periods between irradiation and enucleation, only specimens that were enucleated >30 months after irradiation had no mitotic figures.32 Rapid regression of tumors, particularly taller tumors, has been associated with higher rates of metastasis in both proton-irradiated33 and plaque-treated34 patients, suggesting that more aggressive tumors, due to more rapidly dividing cells, are more radiosensitive than smaller tumors, which may exhibit more prolonged intermitotic phases.
prior radiation treatment.14 Vascular changes include endothelial cell swelling, decreased lumen size, basement membrane thickening, collapse of sinusoidal vessels, and vessel thrombosis. The typical course of regression begins a few months following irradiation, and tumors may continue to regress years after treatment.31 Complete disappearance of the tumor is unusual (see Fig. 13.8). This protracted pattern of tumor regression has been supported by histologic studies among controlled tumors that were enucleated at varying times after proton therapy; although mitotic figures declined with longer periods between irradiation and enucleation, only specimens that were enucleated >30 months after irradiation had no mitotic figures.32 Rapid regression of tumors, particularly taller tumors, has been associated with higher rates of metastasis in both proton-irradiated33 and plaque-treated34 patients, suggesting that more aggressive tumors, due to more rapidly dividing cells, are more radiosensitive than smaller tumors, which may exhibit more prolonged intermitotic phases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree