Chapter 2 Fair Game
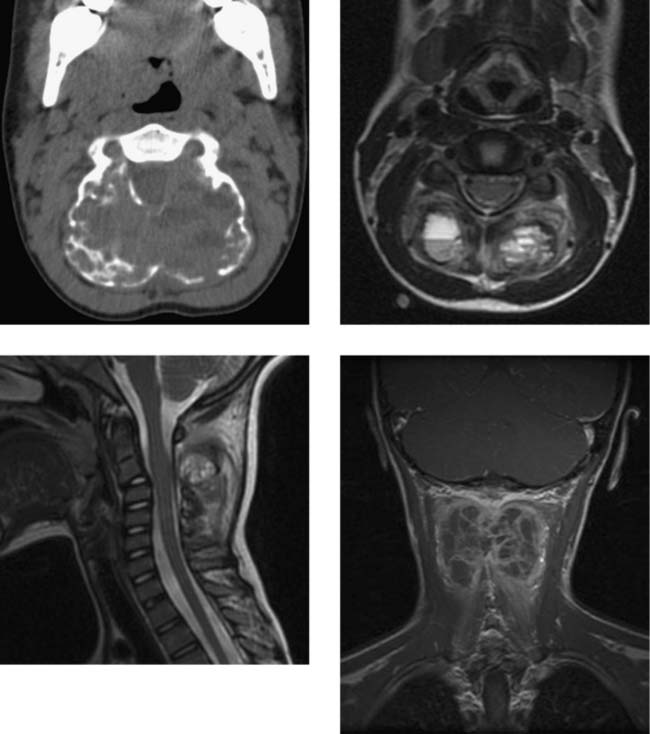
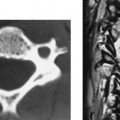
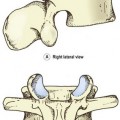
Aneurysmal Bone Cyst (C2)
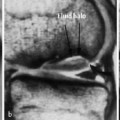
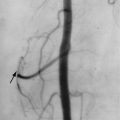
Dural Arteriovenous Fistula
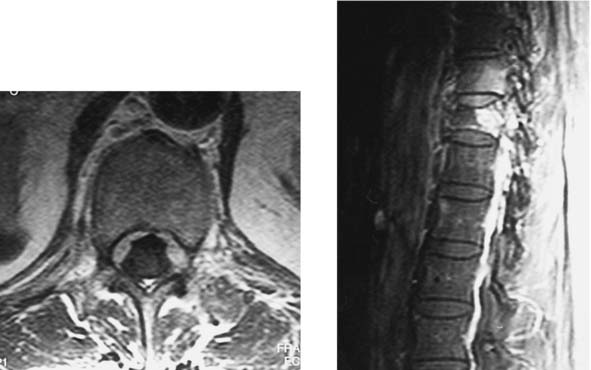
Sickle Cell Disease with Osteomyelitis and Epidural Abscess, Lumbar
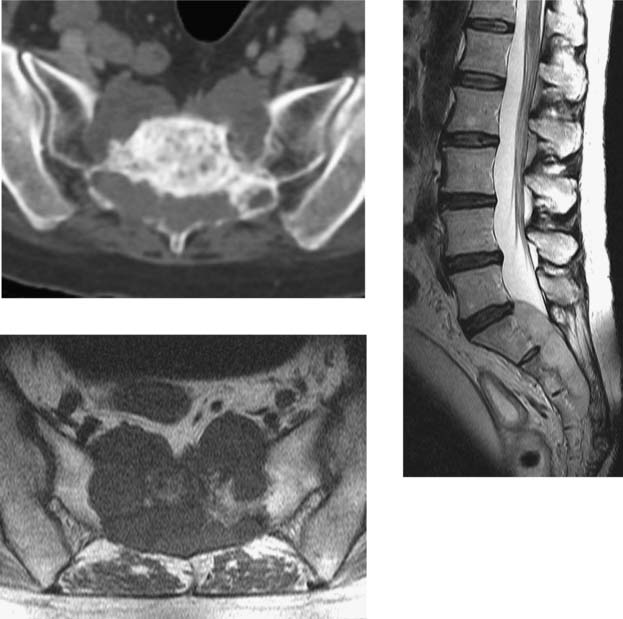
Chordoma, Sacral
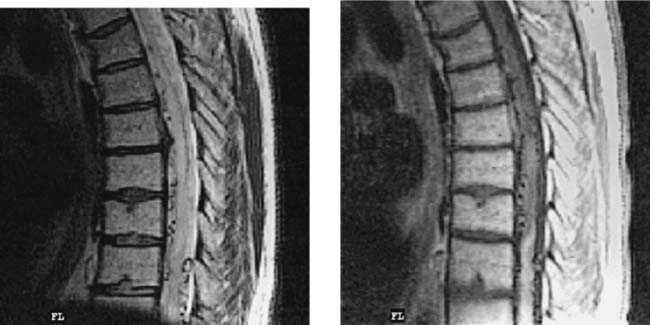
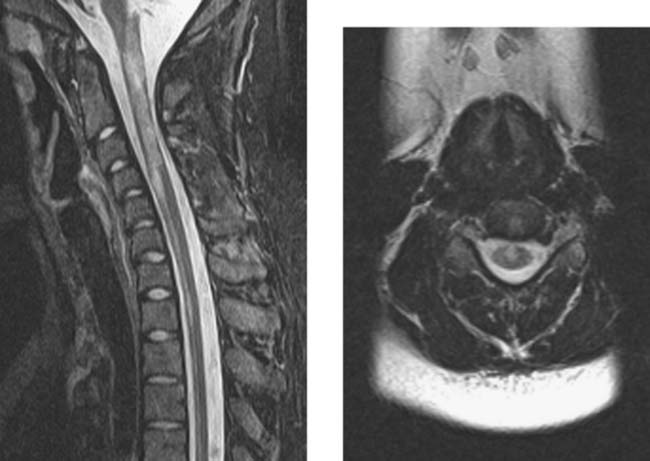
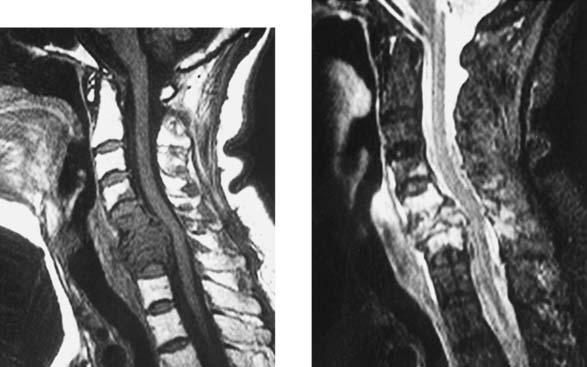
Multiple Sclerosis, Cervical
Clark CA, Werring DJ, Miller DH. Diffusion imaging of the spinal cord in vivo: estimation of the principal diffusivities and application to multiple sclerosis. Magn Reson Med. 2000;43:133-138.
Tartaglino LM, Friedman DP, Flanders AE, Lublin FD, Knobler RL, Liem M. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology. 1995;195:725-732.
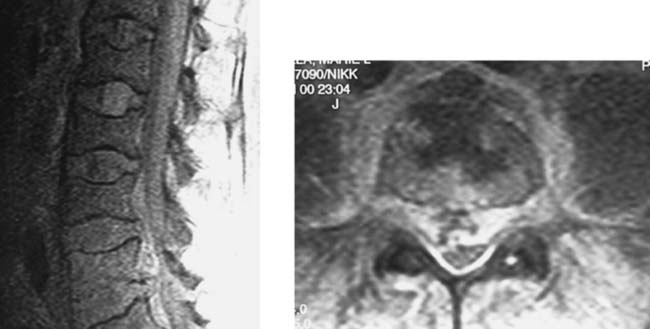
Vertebral Lymphoma with Epidural Venous Enlargement
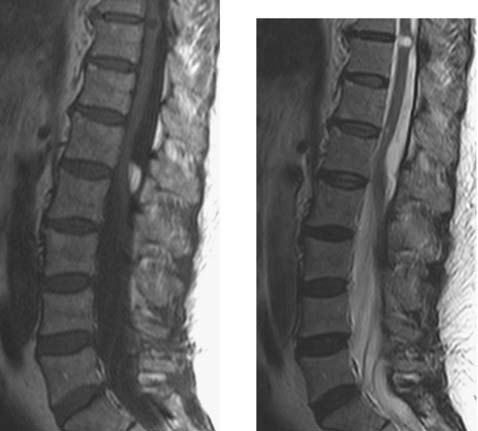
Intradural Lipoma, Lumbar
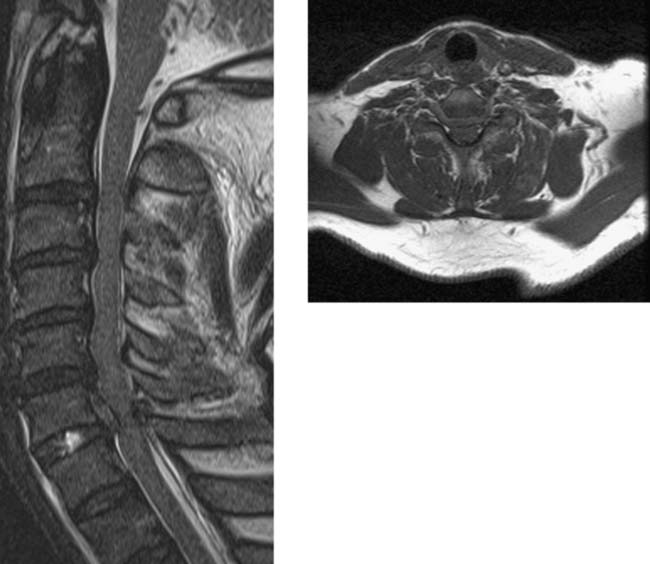
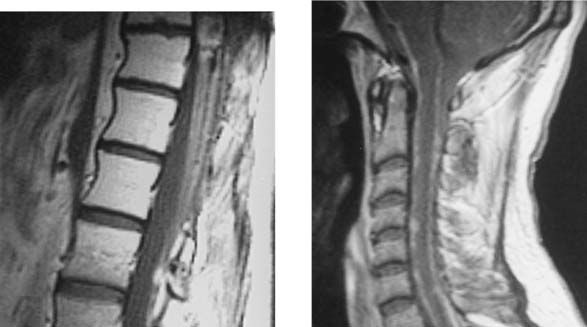
Traumatic Disk Herniation, Cervical
Bucciero A, Carangelo B, Cerillo A, et al. Myeloradicular damage in traumatic cervical disc herniation. J Neurosurg Sci. 1998;42:203-211.
Katzberg RW, Benedetti PF, Drake CM, et al. Acute cervical spine injuries: prospective MR imaging assessment at a level 1 trauma center. Radiology. 1999;213:203-212.
Metastasis Mimicking Diskitis/Osteomyelitis
Gupta RK, Agarwal P, Rastogi H, et al. Problems in distinguishing spinal tuberculosis from neoplasia on MRI. Neuroradiology. 1996;38(Suppl 1):S97-S104.
Kim JK, Ryu KN, Choi WS, et al. Spinal involvement of hematopoietic malignancies and metastasis: differentiation using MR imaging. Clin Imaging. 1999;23:125-133.
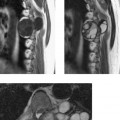
CNS Dissemination of Conus Ependymoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree