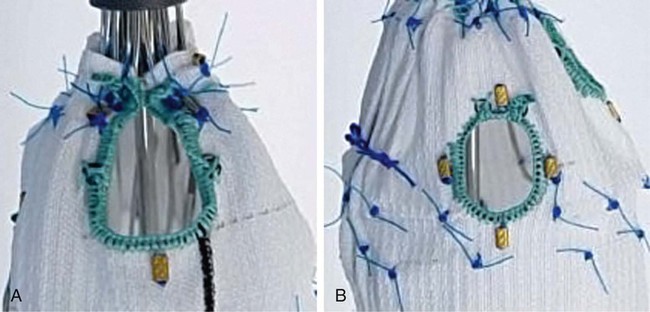
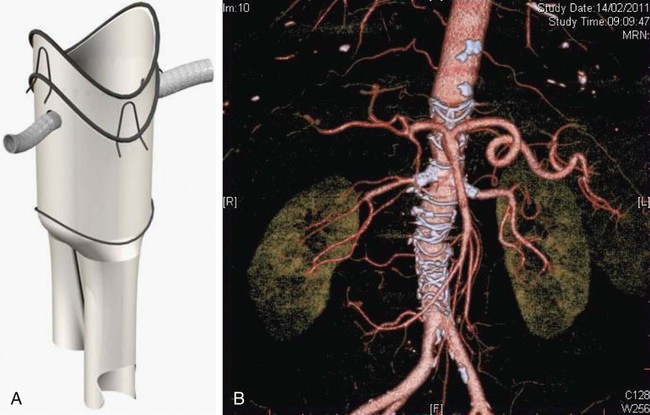
Since the first report of fenestrated endovascular repair of aortic aneurysms (FEVAR) by Park et al. in 1996,1 several large published series and multicenter studies have demonstrated high technical success and promising short- and midterm results. But FEVAR also represents unique challenges to the endovascular interventionalist. These include appropriate patient selection, accurate endograft planning, and demanding technical skills. Clinical indications for FEVAR include aneurysm diameter of 5.5 cm or more, rapidly enlarging aneurysm at a rate of 5 mm over 6 months or 1 cm over 1 year, aneurysm neck 15 mm or less, and patients deemed unfit for open surgery. High-risk patients for major vascular surgery include patients with significant pulmonary disease, poor renal function, severe coronary or heart valve disease, and hostile abdomen.2 There is no absolute contraindication, but the presence of an adverse anatomic feature could make the procedure difficult and increase procedural comorbidity. If there are three or more adverse anatomic features, the procedure could be extremely difficult or impossible.3 Symptomatic or ruptured aneurysm is not suitable for this technology because stent-graft manufacture can take at least 6 weeks. Recent development of off-the-shelf devices may enable their use for symptomatic aneurysms, but it is hard to envisage that ruptured aneurysm would be suitable.4 The proximal component contains two or three fenestrations and a scallop (Fig. 48-1). Rarely, there are four fenestrations. There are two types of fenestrations, small and large. The small fenestration is 6 mm in width and 6 or 8 mm in height. It is reinforced with a nitinol ring and should be 15 mm or more from the proximal edge of the fabric. The large fenestration is 8 to 12 mm in diameter and should be 10 mm or more from the edge of the graft. The scallop is 6 to 12 mm in depth and 10 mm wide. It is also reinforced with a nitinol ring and has gold radiopaque markers. The bare spring (which contains fixation barbs) is located within the top cap. This, together with one or two posterior diameter reducing ties, assists device orientation until final deployment. This fenestrated device was developed on the background of the original Anaconda device.5,6 Its proximal end is positioned suprarenally, and this part of the device provides the seal and fixation by means of hooks and rings. The anterior valley is oriented to cradle the SMA and/or celiac trunk. The fenestrations are made in the unsupported part of the fabric, which gives more flexibility to orient the fenestration according to the circumferential orientation of the renal or SMA vessels. The typical device encompasses two renal fenestrations supported by nitinol rings and marked by four radiopaque markers (Fig. 48-2). However, an SMA fenestration can be added to increase the coverage length in shorter landing zones. Our initial experience with this device shows that potential advantages include the ability to (1) accommodate more difficult anatomy and angulation and (2) partially reposition the top device after cannulating one vessel so that the device can be reoriented, and the next fenestration faces the second vessel. The device also gives the operator the option of cannulating the fenestration antegradely prior to full device deployment, since there is no closed top cap. However, the delivery system profile is still relatively large, similar to the Cook fenestrated stent.
Fenestrated Stent-Grafting of Juxtarenal Aortic Aneurysms
Introduction
Indications and Anatomic Suitability
Contraindications
Devices
New Devices
Custom-Made Fenestrated Anaconda Device (Vascutek, Terumo, Japan)
Radiology Key
Fastest Radiology Insight Engine