Gynecologic cancers impact women of all ages. Some women may wish to preserve their capacity for future childbearing. With appropriate patient selection, acceptable oncologic outcomes may be achieved with preservation of fertility. Determination of eligibility for fertility preservation is guided by patient factors, tumor histology, and preoperative local staging with pelvic MR imaging. The aim of this article is to educate radiologists on the current guidelines for fertility-sparing techniques in women with early stage cervical, endometrial, and ovarian malignancies.
Key points
- •
Some early stage gynecologic cancers may be successfully treated while preserving the patient’s fertility.
- •
Multiparametric pelvic MR imaging plays a key role in determining eligibility for fertility preservation.
- •
Understanding eligibility criteria and how to optimize MR imaging protocols enables radiologists to deliver clinically relevant information and add value with imaging.
Introduction
Many women of reproductive age who are diagnosed with cancer are interested in maintaining their future fertility. For women diagnosed with cervical, endometrial, or ovarian neoplasm, there are various fertility-sparing options for carefully selected patients with early stage cancer. The emerging specialty of oncofertility bridges oncology with reproductive research so that patients have all the necessary information available when making important decisions about future fertility. Approaches may include cryopreservation of eggs or embryos, surrogacy, or organ-sparing surgical techniques. This review focuses on the latter.
If patients are deemed eligible, fertility-sparing options may include conization or trachelectomy for cervical cancer, medical hormonal treatment of endometrial cancer (EC), and cystectomy or unilateral salpingo-oophorectomy for ovarian neoplasm. If pelvic radiation is necessary, ovarian transposition is another option, to relocate the ovaries outside of the radiation field.
Determining which patients are eligible for fertility-sparing treatments relies heavily on accurate cancer staging. When multiparametric MR imaging is performed, the radiologist can answer specific questions about local extent of cervical cancer and EC and can assist in better characterizing sonographically indeterminate adnexal masses. Optimized MR imaging protocols are critical to image the tumors in the appropriate plane and answer the clinically relevant questions for our gynecology/oncology colleagues.
MR imaging protocols
Patients are asked to fast 4 to 6 hours before their MR imaging examination to help reduce bowel peristalsis. Administration of an antiperistaltic agent, such as glucagon, is recommended for all female gynecologic MR imaging. The preferred method of administration is via the intramuscular route, because the medication has rapid absorption and a longer duration (30–60 minutes compared with 10 minutes when using the intravenous route). Additionally, patients should empty their bladder before the scan, because a full bladder can result in motion-related artifact.
A multichannel phased-array receiver coil is used at a field strength of greater than or equal to 1.5 T and patients are imaged in the supine position. Vaginal gel is optional for patients with cervical cancer.
MR imaging sequences may vary across institutions, but there are several key sequences that are integral to improving detection and characterization of masses, such as high-resolution small field-of-view T2-weighted imaging (T2WI). Sagittal high-resolution small field-of-view T2WI is particularly helpful for local tumor staging of cervical cancer and EC. In the setting of cervical cancer, additional high-resolution short-axis oblique T2WI perpendicular to the endocervical canal is performed and high-resolution long-axis oblique T2WI parallel to the endocervical canal is optional. When the clinical indication is for EC staging, the high-resolution short-axis oblique T2WI is performed perpendicular to the endometrial cavity, with optional long-axis oblique T2WI parallel to the endometrial cavity. If the clinical indication is for characterization of an adnexal mass, high-resolution small field-of-view true axial T2WI is performed. Alternatively, some institutions perform an oblique plane T2WI parallel to the endometrial cavity, referred to as the “ovarian axis”, which can assist in visualizing the gonadal vessels and confirming ovarian origin of a mass. ,
Dynamic contrast-enhanced (DCE) MR imaging is a technique where sequential image sets are rapidly acquired through the volume of interest following the intravenous administration of gadolinium contrast. In cases of cervical cancer and EC, DCE MR imaging is acquired in the sagittal plane, with delayed postcontrast T1-weighted imaging (T1WI) in the axial plane. With cervical cancer, DCE MR imaging has been shown to improve tumor-to-cervical stromal contrast in patients with small tumors less than 2 cm in size. , Similarly, DCE is helpful in delineating extent of myoinvasion for staging EC. For adnexal mass characterization, DCE MR imaging is acquired in the axial plane, imaged for a minimum of 3 minutes following contrast administration, and with a temporal resolution less than 15 seconds.
DCE MR imaging is particularly helpful in characterizing sonographically indeterminate adnexal masses. Regions of interest are placed on the most rapidly enhancing component of the ovarian mass and the outer myometrium. The time-intensity curve of the ovarian mass is compared with the myometrial reference, which generally has a steep initial slope and a shoulder. Three unique time-intensity curves have been described in adnexal lesions. A type I curve is defined as slow progressive enhancement without plateau and is suggestive of benignity. A type II curve demonstrates an initial slope less than that of myometrium but with a distinct shoulder, and has been associated with intermediate-risk lesions, such as borderline ovarian tumor. A type III curve demonstrates a rapid initial wash-in with a slope that is greater than adjacent myometrium, suggestive of probable malignancy.
When high temporal resolution DCE is not available, a multiphasic acquisition obtained at intervals of 30 seconds or more may still provide useful information about enhancement kinetics. When a mass enhances less than the myometrium (or much less than pelvic arteries) in the arterial phase, an aggressive ovarian malignancy is unlikely.
Cervical cancer and fertility-sparing options
Cervical cancer is commonly diagnosed in women of reproductive age, typically attributed to sexual activity and transmission of the human papilloma virus infection, a strongly associated risk factor. In a select patient population, fertility-sparing techniques may be applied, to allow for future fertility in these women.
Cone biopsy, or conization, refers to the en bloc removal of the squamocolumnar junction, which includes the ectocervix and endocervical canal. This technique may constitute definitive treatment of patients with stage IA1 tumors with negative margins and absence of lymphovascular space invasion. Patients with stage IA1 tumors with lymphovascular space invasion or stage IA2 tumors require lymph node assessment in the form of either pelvic lymphadenectomy or pelvic sentinel lymph node biopsies in conjunction with conization, radical trachelectomy, or hysterectomy. Stage IA tumors are not visible at MR imaging and are diagnosed by microscopy.
Radical trachelectomy is performed vaginally or abdominally, via an open or minimally invasive approach. The operation involves surgical removal of the cervix, the parametria, and the upper vagina. The upper vagina is then sutured to the lower uterine body. A cerclage or “purse string” stitch is placed by the surgeon in the lower uterine body to prevent pregnancy loss.
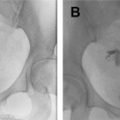
There are specific eligibility criteria ( Table 1 ) required for consideration of radical trachelectomy for fertility-sparing treatment of cervical cancer, but individual practice patterns vary. Patients must desire fertility preservation and the preoperative evaluation must suggest that the patient is at low risk for needing postoperative radiation therapy. The tumor must be confined to the cervix, should be less than or equal to 2 cm in size, and the superior aspect must be greater than or equal to 1 cm from the internal os ( Fig. 1 ), although tumors 2 to 4 cm are not an absolute contraindication. Histology should be squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Broadly, exclusion criteria include tumor size greater than 2 cm, parametrial spread, lymphovascular space invasion, and nodal or distant metastatic disease.
| Gynecologic Cancer | Inclusion/Eligibility Criteria | Exclusion Criteria |
|---|---|---|
| Cervical | Tumor confined to cervix Tumor ≤2 cm in size Distance ≥1 cm between tumor and internal os Cervical stromal invasion <50% Favorable histology (squamous cell carcinoma, adenocarcinoma, adenosquamous) | Parametrial spread Tumor >2 cm in size Distance <1 cm between tumor and internal os Cervical stromal invasion >50% Lymphovascular space invasion (pathology diagnosis) Nodal or distant metastatic disease Aggressive histologic subtypes (eg, neuroendocrine tumor, adenoma malignum) |
| Endometrial | Absence of myometrial and cervical invasion No genetic predisposition for ovarian cancer Favorable histology (grade I endometrioid adenocarcinoma or premalignant condition) | Synchronous primary ovarian cancer or ovarian metastatic disease Nodal or distant metastatic disease Aggressive histologic subtypes (grade 2 and 3 endometrioid adenocarcinoma, serous, clear cell) |
| Ovarian | Any stage malignant germ cell tumor, sex-cord stromal tumor, or borderline tumor Stage I epithelial cell tumor Absence of extraovarian disease | Nodal or distant metastatic disease |

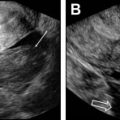
Tumor size, location relative to the internal cervical os, parametrial extension, and nodal metastases can all be assessed with pelvic MR imaging. On T2WI, cervical cancer typically appears as an intermediate signal intensity lesion that may or may not disrupt the low-signal intensity cervical stroma. Most tumors are either isointense or hypointense relative to adjacent stroma on the delayed postcontrast imaging. Small tumors may enhance earlier than adjacent cervical stroma, which is better delineated with DCE MR imaging. Cervical tumors typically demonstrate high signal intensity on high-b-value diffusion weighted imaging (DWI) with corresponding low signal on the apparent diffusion coefficient maps, relative to cervical stroma ( Fig 2 ).

Tumor size should be reported in all three dimensions, with craniocaudal axis relative to the endocervical canal and distance from the internal os. At least 1 cm length between the superior margin of the cervical tumor and the internal os is desired, to allow for a negative margin and safe surgical anastomosis. Some institutions have decreased this minimum length to 0.5 cm. At MR imaging, the internal os anatomic landmark is defined as the waist of the uterus, the point at which the low signal intensity cervical stroma becomes intermediate signal intensity myometrium. The internal os is also the entrance site of the uterine vessels ( Fig. 3 ). A cervical tumor that extends to or involves the internal os is ineligible for fertility-sparing techniques ( Fig. 4 ).


Many patients may have a history of prior cone biopsy or other excisional procedure, which may affect the cervical length. The total length of the cervix should ideally be greater than 2.5 cm. Additional cone biopsy may be deferred in patients with a short cervix, given potential future complications of cervical incompetence and preterm delivery. , ,
At T2WI, cervical stromal invasion is present when the intermediate signal intensity tumor disrupts the low-signal intensity cervical stroma. Deep stromal invasion is defined as a cervical tumor that invades the outer third of the cervical stroma ( Fig. 5 ). Given association between deep stromal invasion and higher recurrence rate and shorter survival, ideal tumors for a fertility-preserving approach involve less than half of the stromal thickness (see Fig. 1 ). , Because this parameter is difficult to assess clinically and by MR imaging, preoperative suspicion of deep stromal invasion is a relative rather than absolute contraindication and is balanced against other factors in surgical decision-making. ,

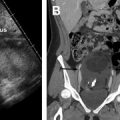
By contrast, parametrial invasion is a definite contraindication to radical trachelectomy and denotes at least stage IIB disease. At MR imaging, parametrial invasion is present if the tumor disrupts the low signal intensity cervical stromal ring ( Fig. 6 ). Lymph node or distant metastases are also a contraindication to fertility-preserving surgery. Pelvic or para-aortic lymph nodes larger than 1 cm in short axis are suspicious. Additional morphologic features concerning for nodal metastatic disease include irregular borders, loss of fatty hilum, presence of necrosis, and signal intensity that is similar to the primary tumor. Both computed tomography and MR imaging are limited in detecting metastatic disease in normal-sized lymph nodes, but fluorodeoxyglucose PET is often used clinically to assess for extracervical disease.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree