Fig. 1
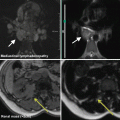
A 46-year-old male with acute abdominal symptoms, unenhanced abdominal CT shows perforated diverticulitis with free abdominal gas (not shown). Incidentally, a right renal mass, isodense with renal parenchyma, was noted (arrow). Follow-up with contrast-enhanced CT showed clear cell renal carcinoma, histologically confirmed at surgery
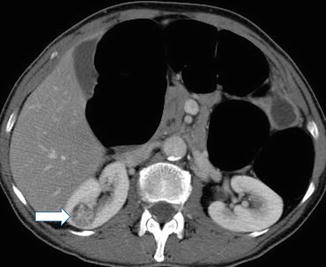
Fig. 2
A 61-year-old woman with bowel symptoms examined with CT colonography. On the supine, contrast-enhanced series (above), a 2 cm solid, diffusely contrast-enhancing tumor is noted in the right kidney (arrow). This lesion was not detectable on the prone, non-enhanced series, as it was isodense with normal parenchyma and not exophytic. Surgical removal showed clear cell renal carcinoma
5.2 Benign Renal Lesions
As mentioned above, in most cases, benign renal neoplasms cannot reliably be differentiated from malignant ones on non-contrast- or contrast-enhanced CT. Thus, oncocytomas, which are benign, may simulate renal cancer on CT (Fig. 3), and even at biopsy, it may sometimes be impossible to differentiate the two. Many of these tumors therefore go to surgery or percutaneous ablation without a definite diagnosis but with the chance of being malignant in 85–90% of the cases. All incidentally detected solid tumors in the kidneys should thus be considered potentially malignant and be fully investigated as such. One exception, however, is renal angiomyolipoma (AML), which is a benign tumor containing vascular, muscular, and fatty tissue components in varying proportions. In most cases, the fatty component is dominant or at least abundant enough to make it readily identifiable on non-contrast-enhanced CT (Fig. 4). Identification of macroscopic fatty components in regions of interest (density below −10 HU and preferably lower) is virtually diagnostic of AML (Jinzaki et al. 2014). Although these tumors are benign, they may occasionally show (benign) involvement of local lymph nodes. As most AMLs are asymptomatic, they are usually detected incidentally. Although these tumors are commonly clinically silent, with growth, there is a risk of bleeding, which may be acute and severe. Therefore, if an AML is 4 cm or larger, preventive embolization, ablation, or surgical removal is often considered. This means that incidentally detected AMLs smaller than 4 cm should be followed up in order to estimate their growth potential. Such follow-up is best performed with CT or MRI, which provide more reproducible size measurements than ultrasonography.



Fig. 3
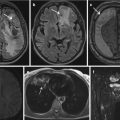
Incidentally detected solid, renal mass in the posterior part of the left kidney (arrow). Subsequent surgical removal showed oncocytoma

Fig. 4
Angiomyolipoma (AML) in the posterior part of the right kidney (long arrow), incidentally detected on acute non-enhanced abdominal CT in a 75-year-old woman with abdominal pain. The fatty components (mean − 45 HU) are characteristic for AML. The maximum diameter of the lesion was 7 cm, and due to the risk of spontaneous bleeding, the lesion was embolized. Note also faintly calcified stones in the normal-sized gallbladder (short arrow)
Occasionally, the fatty component of an AML is minimal and not readily identifiable on CT. Although fatty components may be identified by analysis on pixel level, such “fat-poor” AMLs may simulate renal cell carcinoma. Fat-poor angiomyolipomas may be hyperattenuating relative to renal parenchyma on non-enhanced CT with density measurements >45 HU, or, rarely isoattenuating and contrast enhancing, similar to some renal cell carcinomas. In questionable cases, MRI may be of help to demonstrate or rule out a fatty component (Jinzaki et al. 2014). Renal cancers do not exhibit fatty content, unless the tumor engulfs normal fatty tissue in the renal sinus, which has been described in rare cases.
If angiomyolipomas are detected at a young age, or if large, multiple, or bilateral, tuberous sclerosis should be suspected, as angiomyolipomas develop in over half of patients with tuberous sclerosis. Angiomyolipomas in patients with tuberous sclerosis seem to grow faster and may be more prone to bleeding and may therefore need treatment, including mTOR inhibitors, in a higher proportion than sporadic angiomyolipomas (Jinzaki et al. 2014).
5.3 Small Lesions
The risk of a solid renal mass lesion being malignant increases with the size of the lesion (Thompson et al. 2009). As pointed out above, solid renal masses tend to be small when detected incidentally. However, it is uncertain to what extent really small renal lesions (<1 cm) are reported by radiologists. Some subcentimeter lesions visually stand out as clearly low density compared to the surrounding enhancing parenchyma, suggesting a cystic character. However, objective measurements of density (HU numbers), to confirm cystic or solid nature of such small lesions, are problematic. This may be related to technical factors such as slice thickness, kilovoltage and amperage settings, contrast medium dose and timing, partial volume effects, and particularly pseudoenhancement due to beam hardening. Pseudoenhancement is more prone to occur with small (<1.5 cm) and centrally located lesions surrounded by contrast-enhancing renal parenchyma, while it is less apparent in larger lesions and in lesions with peripheral location (Tappouni et al. 2012; Patel et al. 2014). The risk of misinterpreting the nature of small renal lesions due to these factors should thus be considered. Commonly, 15 HU or even 10 HU increase in density after intravenous contrast injection, as compared to the native series, has been used to classify lesions as enhancing, thereby calling them solid. However, there is no consensus regarding the optimal cutoff, and lately even 15–20 HU enhancement has been considered indeterminate. In a recent study, the post-contrast-enhancement pattern in 137 verified solid renal tumors (85% malignant and 15% benign) measuring 1.0–3.9 cm (median 2.4 cm) was analyzed (Al Harbi et al. 2016). Using 15 HU post-contrast enhancement to define a mass as solid, 17% of the malignant lesions did not reach the threshold in the corticomedullary phase, 8% did not reach the threshold in the nephrographic phase, and 3% did not reach the threshold in both the corticomedullary and the nephrographic phases. Using 20 HU as the threshold, 21% of the malignant lesions did not reach the threshold in the corticomedullary phase, 12% did not reach the threshold in the nephrographic phase, and 9% did not reach the threshold in both phases. In particular, papillary cancers did not reach the 15 HU or 20 HU threshold in over half of the cases in the corticomedullary phase, while the corresponding figures in the nephrographic phase were 18% (15 HU threshold) and 32% (20 HU threshold). About a third of the chromophobe cancers did not reach the thresholds in any phase. Even the clear-cell cancers did not reach the 15 HU threshold in 11% (corticomedullary phase) and 7% (nephrographic phase), while the combination of corticomedullary and nephrographic phases reduced the proportion of clear-cell cancers not reaching the 15 HU and 20 HU thresholds to 5% and 6%, respectively. All of the benign lesions had post-contrast enhancement exceeding both thresholds in all phases (Al Harbi et al. 2016). It can be concluded that applying the 15 HU or 20 HU threshold on both the corticomedullary and nephrographic phases results in the best sensitivity for classifying a lesion as solid or not. Even so, benign and malignant renal tumors in most cases cannot be reliably separated on the basis of their enhancement pattern. Although most small renal cancers enhance above these thresholds with a wide margin, the fact that some do not enhance above 15 HU or 20 HU may pose a problem to differentiate e.g. a hyperdense cyst from a solid tumor. For indeterminate lesions, contrast-enhanced ultrasound or MRI should therefore be considered for problem-solving.
Lesion enhancement after contrast medium administration is a cornerstone in the differentiation between solid and cystic lesions, but other factors such as lesion demarcation, homogeneity, and occurrence of necrosis and calcifications must be taken into consideration. Reporting and decision-making must also take the clinical situation, especially the age of the patient and comorbidity, as well as the potential tumor growth potential, into consideration in order to avoid false-positive cases leading to unnecessary further examinations. If a subcentimeter lesion does not show any obvious malignant characteristics but is too small to characterize further by imaging, it is comforting that such small lesions are very unlikely to be malignant at the time (Berland et al. 2010). Even if a 1-cm renal tumor is malignant, it is very unlikely to have metastases at presentation (Thompson et al. 2009). Unless the patient is young and has a genetic risk or renal tumor is specifically searched for (which is not the case with an incidental finding), aggressive follow-up for further characterization of subcentimeter lesions is not generally recommended (Hindman 2015).
5.4 Cystic Renal Lesions
It is commonly stated that simple renal cysts occur in 50% of individuals over 50 years of age, based on autopsy findings. On abdominal CT, benign renal cysts are one of the commonest incidental findings (Carrim and Murchison 2003). There is a clear increase in the frequency and number of renal cysts with increasing age. Thus, cysts are rarely present under the age of 40 years (found in 8% of the patients), while it was found in 61% of patients aged over 80 years (Carrim and Murchison 2003). If multiple renal cysts occur in patients under 40 years of age, it may be indicative of autosomal dominant polycystic kidney disease (ADPKD) (see below). As simple renal cysts virtually always are symptom-free, they are nearly always incidental findings. Very rarely a large simple cyst may be suspected to cause pain or discomfort, and in such exceptional cases, a diagnostic percutaneous puncture and emptying of the cyst fluid may show if the cyst is the cause of the problem. After such drainage, the cyst usually refills in a short time, so if symptomatic and needing treatment, the cyst could be treated by surgical de-roofing.
The challenge for the radiologist when evaluating renal cyst-like lesions is to differentiate simple, benign cysts from atypical complex cysts and cystic tumors, which may require additional imaging or follow-up.
5.5 Simple Cysts
Benign simple cysts are characterized by a round or oval shape, low-density, homogeneous fluid content typically measuring <20 Hounsfield units (HU), and thin wall. After IV contrast injection, they should remain low in density, with less than 10–15 HU increase. However, one must consider that pseudoenhancement may occur, as discussed above. Most incidentally detected renal cysts can be easily dismissed on contrast-enhanced CT, based on the criteria above. A cyst which is well demarcated, thin walled, of low, homogeneous density, and without septa, solid parts, or calcifications should be called and reported as a benign cyst and does not require follow-up, regardless of the size of the cyst.
Renal cysts of benign appearance may also occur with a number of underlying specific disorders, which may be incidentally encountered on abdominal CT performed for various reasons. In patients on long-standing lithium therapy, renal dysfunction may develop, including a large number of small (1–2 mm), bilateral, cortical, and medullary “microcysts” in normally sized kidneys (Wood et al. 2015). Another cause of acquired cysts is end-stage renal disease and dialysis, which commonly are associated with the development of renal cysts (defined as at least three cysts in each kidney, usually in small, atrophic kidneys). This type of acquired cystic kidney disease is associated with occasional cyst bleeding and an increased risk of renal cancer development (Katabathina et al. 2010).
Occasionally, an unexpectedly large number of renal cysts in normal sized or enlarged kidneys are incidentally noted on abdominal CT. If this occurs in young or middle-aged patients, it may indicate autosomal dominant polycystic kidney disease (ADPKD). This is characterized by enlarged kidneys with multiple bilateral renal cysts, which develop and increase in number and size with age (Pei et al. 2015). The multitude of bilateral renal cysts may be accompanied by liver cysts, sometimes causing a considerable mass effect and occasionally pancreatic and other cysts (Kim et al. 2015). As the disorder is familial, most patients are aware of their potential disease at an early stage, but sometimes the diagnosis is first suspected at cross-sectional imaging in young or middle-aged adults, by incidental detection of multiple renal cysts. Normally, renal cysts are rarely detected in individuals under 30 years of age. APKD should be suspected if three or more cysts are found in one (or both) kidneys in patients under 40 years of age, two or more cysts in each kidney in patients 40–59 years, or four or more cysts in each kidney in patients aged 60 or more (Pei et al. 2009).
5.6 Complex Cysts
Cysts which do not fulfill the criteria for simple cysts are called complex cysts. These constitute a considerable part of incidentally detected cysts and cause considerable concern for radiologists and clinicians. Complex cysts are characterized by one or several of the following features: higher than expected density for a simple cyst (>20 HU), localized or global wall thickening, and internal septations, calcifications, or a solid component in a predominantly cystic lesion. Complex cysts may be entirely benign, but at the other end of the spectrum are cystic malignant tumors and cyst-like necrosis in malignant tumors. These latter cystic lesions may be easy to identify when they contain a clearly solid, contrast-enhancing component, and the concern is mainly about those that exhibit some of the above features, without convincing evidence of malignancy.
One variant of complex cyst often detected incidentally is the protein-rich or hemorrhagic cyst (Fig. 5). These are cysts of high, homogeneous density above 20 HU on non-enhanced CT, without significant increase (<15 HU) in density after intravenous contrast administration and without any other features of complex cysts (i.e. absence of calcifications, septations, wall thickening, and solid components). As with any HU cutoff, there is overlap between normal and abnormal cyst density, variations depending on the choice of image slice and size and placement of the region of interest (ROI) as well as inherent variations between CT machines (Hammarstedt et al. 2013). As discussed above, HU cutoffs should be considered as rule of thumbs to be applied sensibly, taking all imaging characteristics into consideration.


Fig. 5
Incidental detection of a 12 mm hyperdense exophytic renal lesion with homogeneous density of 67 HU on non-enhanced CT. After intravenous contrast injection, the density was unchanged. The finding is characteristic for cyst with high-protein content (hemorrhagic cyst)
Cysts may be rich in protein due to bleeding or infection, although the etiology cannot be proven in most cases. For example, in autosomal dominant polycystic kidney disease with a large number of cysts, the conversion of simple cysts to high-density cysts from one examination to another is not unusual. This is frequently interpreted as cyst bleeding, which usually is symptom-free, although it may occasionally be associated with pain. If a hyperdense renal lesion is incidentally detected on non-contrast-enhanced CT, differentiation between a hemorrhagic cyst and solid tumor should be affirmed by contrast-enhanced CT, MRI, or ultrasonography.
5.7 Bosniak Classification
Incidentally detected cysts which exhibit features of complexity are best classified by the Bosniak classification system. Originally presented in 1986 (Bosniak 1986), this system allows categorization of renal cysts according to the degree of complexity (Bosniak I–IV) and also provides recommendations on follow-up. Because of difficulties in separating Bosniak II and III, an additional category, Bosniak IIf (f for follow-up), was added (Israel and Bosniak 2003). The categorization is based on the cyst fluid density, post-contrast enhancement characteristics, degree of wall thickness, occurrence of internal septations and calcifications, and enhancing soft tissue nodules. A simple cyst is classified as Bosniak I if of water density, not contrast-enhancing, thin walled, and without septations, calcifications, or solid components (Fig. 6). Bosniak II cysts are characterized by “a few hairline-thin septa, fine calcification, or a short segment of slightly thickened calcification present in the wall or septa (Fig. 6). Uniformly, high-attenuation lesions (<3 cm) that are sharply marginated and do not enhance are included in this group.” Bosniak II cysts are also considered to be benign. Bosniak IIf cysts exhibit somewhat more complexity: “These cysts may contain an increased number of hairline-thin septa. Minimal enhancement of a hairline-thin smooth septum or wall can be seen, and there may be minimal thickening of the septa or wall. The cyst may contain calcification that may be thick and nodular, but no contrast enhancement is present. There are no enhancing soft-tissue components. Totally intrarenal nonenhancing high-attenuation renal lesions that are 3 cm or larger are also included in this category. These lesions are generally well marginated.” The recommendation for Bosniak IIf is to follow these lesions and to determine change in size or character.


Fig. 6
Examples of Bosniak I, IIF, and III classification of cystic renal lesions. Note the solid, contrast-enhancing elements of the Bosniak III lesion. As additional incidental finding, a mass in the bladder, suggestive of enlarged prostate, is noted
Bosniak III cysts are defined as follows: “These lesions are indeterminate cystic masses that have thickened irregular walls or septa in which enhancement can be seen.” Bosniak IV: “These lesions are clearly malignant cystic masses that not only have all the characteristics of category III lesions, but also contain enhancing soft-tissue components adjacent to but independent of the wall or septa” (Israel and Bosniak 2003) (Fig. 6).
It may be difficult to understand the details of the Bosniak classification by just reading the definitions. The classification system is better understood by looking at the clinical case illustrations presented in Bosniak’s own original articles (Bosniak 1986, Israel and Bosniak 2003). Although not perfect in its prediction of malignant development, the Bosniak classification system offers a good help when complex cysts are incidentally encountered, including advice on follow-up. Decision on follow-up recommendations should be based on the Bosniak classification, but the patient comorbidity, age, and patient’s own preferences must also be taken into consideration.
5.8 Renal Calcifications
Incidental renal calcifications are common, especially in the elderly. On unenhanced CT, even very small calcifications (1–2 mm) are easy to detect. When encountering a renal calcification, the following question should be asked: Does the calcification represent a urinary stone (located in a calyx, the renal pelvis, or ureter), a parenchymal calcification, or a vascular calcification? Vascular (arterial) calcifications are usually easy to identify by their location close to the renal hilum and in the course of the renal artery, and the finding may be supported by the coexistence of other vascular calcifications suggesting generalized atherosclerosis. In older patients with generalized vascular calcifications, renovascular calcifications are not commonly reported by the radiologist, as vascular calcifications can be considered as part of normal aging. However, in young patients, and in older patients with advanced calcifications, it might be worthwhile to report, as it may be related to treatable renal artery stenosis and renovascular hypertension (Glodny et al. 2012).
It may sometimes be difficult to differentiate a parenchymal calcification from a stone in the collecting system on non-enhanced CT and on CT obtained in the cortical or nephrographic phase, when there is not yet contrast medium filling of the collecting system, making it difficult to outline. This is rarely a problem in the excretory phase, when the collecting system is well depicted, although urinary stones may be hidden in the contrast-filled collecting system. Parenchymal calcifications are relatively rare and may be related to, e.g., nephrocalcinosis, tubular necrosis, tuberculosis, or other infections and sometimes to renal carcinoma. In case of tuberculosis, however, there are usually other typical manifestations such as corresponding parenchymal thinning and calyceal strictures and dilatation or tuberculosis manifestations in other organs. With renal carcinoma, calcifications rarely occur in small tumors, while larger calcified tumors usually are evident by their space-occupying characteristics.
Any calcifications suspected to be stones located in the collecting system should be reported, as they may potentially be displaced to the ureter causing obstruction. Even if small and not likely to cause pain or obstruction when located in a calyx, they may be of importance. Thus, they may increase in size with time, and the patient may benefit from early detection, follow-up, and perhaps treatment with extracorporeal shock wave lithotripsy (ESWL).
5.9 False-Positive Renal Masses
Focal compensatory hypertrophy associated with post-pyelonephritic parenchymal scar formation may sometimes simulate a renal mass lesion, although scar formation is more often associated with parenchymal atrophy, rather than giving an impression of mass lesion. As scar formation is a long-term effect of previous acute infection, scars may be encountered in symptom-free patients as incidental finding on CT. If in doubt, calyceal clubbing corresponding to the site of parenchymal scar formation should be looked for, to support post-pyelonephritic scarring, which is also characterized by multifocal, asymmetrical distribution in the kidney. This is different from persisting fetal lobulation, where smooth indentations of the renal outline are seen not opposite but between the pyramids. Another potential pitfall is hypertrophy of a column of Bertin, a normal variant occasionally interpreted as a renal tumor. A column of Bertin (columna renalis) represents normal cortical tissue extending deep into the kidney from the peripheral cortex, having exactly the same post-contrast attenuation as the rest of the renal cortex (Ramanathan et al. 2016).
5.10 Renal Size
The size of the kidneys should always be assessed, taking normal parenchymal thinning with age into consideration, and discrepancies in size of the two kidneys should be mentioned in the radiology report.
5.11 Normal Variants and Malformations
Among other clinically relevant incidental findings on abdominal CT, normal variants and malformations of potential clinical importance should be mentioned. Thus, congenital absence of a kidney or status post nephrectomy (single kidney) should be documented, as it may otherwise lead to confusion if the patient later undergoes, e.g. abdominal ultrasonography. Also, this information is of clinical value because of the risk of hyperfiltration and subsequent glomerulosclerosis that may occur after nephrectomy (Abdi et al. 2003). Likewise, duplication of the collecting system, ectopic and malrotated kidneys, and horseshoe kidney (Fig. 7) should be mentioned (Ramanathan et al. 2016). A horseshoe kidney is a renal fusion anomaly with functioning renal parenchyma or fibrotic tissue bridging the midline and the two renal units. Horseshoe kidneys usually have multiple renal arteries, sometimes originating from the distal aorta or iliac arteries, of importance in case of surgery or interventional procedures. Horseshoe kidneys occur in approximately 1/500 adults and are usually asymptomatic. However, they carry an increased risk for obstruction, infection, and stone formation, and it may be vulnerable in abdominal trauma. In some cases, horseshoe kidney can be linked to other malformations or a variety of genetic or other syndromes and to an increased risk of malignancy.


Fig. 7
Incidentally detected horseshoe kidney in a woman who had an arterial phase CT because of suspected aortic dissection. It was revealed that the patient had Turner’s syndrome, which carries an increased risk of renal fusion anomaly (horseshoe kidney)
5.12 Hydronephrosis
Incidental detection of hydronephrosis and hydroureter, which may indicate urinary tract obstruction, should be mentioned. In such cases, it should be determined if it is uni- or bilateral, if it is associated with urteral dilatation, and if it is associated with generalized parenchymal thinning, which suggests more long-standing obstruction. Although hydronephrosis is usually related to urinary obstruction, this is not always the case, as dilatation may remain permanently after removal of an obstruction, if the obstruction has been longstanding and the system thereby lost some of its elasticity. Hydronephrosis on the basis of obstruction is associated with dilatation of the renal pelvis as well as calyces. It should be differentiated from a normal but large extrarenal renal pelvis without calyceal dilatation, which is not indicative of obstruction. If the CT is done with IV contrast administration, the function of the parenchyma and, with delayed scan in the excretory phase, the urinary outflow may be assessed. Another pitfall on non-enhanced and early post-contrast scanning is the existence of peripelvic cysts, which also may simulate hydronephrosis. However, in the excretory phase, differentiation between hydronephrosis and a cluster of peripelvic cysts is usually straightforward (Fig. 8). Less commonly, a parapelvic cyst, i.e. an ordinary cyst originating from the renal parenchyma and extending into the renal sinus region, may be mistaken for hydronephrosis.


Fig. 8
Incidental finding suggestive of hydronephrosis on contrast-enhanced abdominal CT in the corticomedullary phase, before iodine contrast material arrives in the collecting system (upper row: coronal (a) and axial (b) planes, respectively). Images obtained a few minutes later (in the excretory phase) clearly show that the collecting system has normal width (lower row: coronal (c) and axial (d), respectively) and that the hypodense fluid-containing structures represent peripelvic cysts. Peripelvic cysts are not uncommon and are claimed to develop from lymphangiectasia, in contrast to parapelvic cysts which represent ordinary cysts protruding into the sinus region
6 Urinary Bladder and Upper Urinary Tract Tumors
The urinary bladder has traditionally been the domain for the urologists, cystoscopy being the primary method for tumor detection. However, improved quality of CT allows detection of bladder tumors in many instances (Raman and Fishman 2014). The vast majority of patients with bladder or upper urinary tract cancer present with hematuria, and the workup includes cystoscopy and CT urography. The frequency of incidentally detected bladder and upper urinary tract cancers is largely unknown but appears to be low.
Unless grossly space occupying, bladder tumors are best visualized in the corticomedullary phase, as compared to the nephrographic and excretory phases (Helenius et al. 2016), due to their high attenuation in the arterial phase. As early detection of bladder cancer may improve prognosis, the bladder should routinely be scrutinized for incidental tumor detection, especially in middle-aged and older individuals, having in mind the better chance of tumor detection on contrast-enhanced CT series. Nevertheless, many bladder tumors can be depicted also on non-enhanced CT (Fig. 9).


Fig. 9
Two centimeter rounded bladder wall tumor (arrow), hyperdense relative to the urine and protruding into the bladder lumen, on non-enhanced CT
Tumors of the calyces, renal pelvis, and ureters are much less common than urothelial bladder tumors, representing about one tenth of the total number of urothelial tumors. Thus, they are relatively rare tumors, not commonly detected as incidental findings. Typical findings at careful assessment of the collecting system and ureters are wall-thickening and contrast-filling defects on images obtained in the excretory phase, with or without dilatation depending on the degree of outflow obstruction (Xu et al. 2010). The nephrographic phase has been shown to demonstrate upper urinary tract tumors in a higher frequency compared to the excretory phase (Metser et al. 2012), but the combination of the two provides a better diagnostic accuracy. However, as for bladder cancer, the best possibility for incidental detection of upper urinary tract tumors appears to be in the corticomedullary or arterial phase.
7 Adrenals
Adrenal masses are among the most common incidental findings on CT of the abdomen. Hammarstedt et al. found a frequency of 4.5% in a reevaluation of 3,801 unselected clinical abdominal CT examinations, from a cohort of over 30,000 CT examinations (Hammarstedt et al. 2010). The same study showed a considerable variation in the frequency of reported lesions between hospitals (range 1.8–7.1%), suggesting considerable under-reporting in clinical practice, although differences in patient population profiles and other factors also may be a factor. The frequency of adrenal incidentalomas increases with age. Figures from autopsy studies suggest figures in the range of 7–8% (Abecassis et al. 1985) or even higher in the elderly, depending on diagnostic criteria used and the age and character of the studied populations. The vast majority of adrenal incidentalomas are non-hyperfunctioning adenomas, but the task of the radiologist is to determine, with reasonable certainty, if the lesion is a benign adenoma, cyst or other benign lesions, or malignant primary or metastatic tumor.
When an unexpected adrenal lesion is identified on CT, three questions should be raised: First, does the patient have a known malignancy? Second, does the lesion have benign, indeterminate, or malignant CT characteristics? Third, is the lesion hyperfunctioning or not?
The first question – does the patient have a known malignancy – is very relevant as the risk of an incidentally detected adrenal mass being malignant is very low if the patient has no known malignancy. Thus, Song et al. (2008) found no case of malignant adrenal lesion in 1,049 adrenal incidentalomas in patients without malignant disease. In a patient with known malignancy, on the other hand, an adrenal mass may represent a metastasis or an unrelated benign lesion. In patients with a previous history of extra-adrenal malignancy, incidentally detected adrenal lesions were found to be benign in 74% of the cases. In patients with concurrent extra-adrenal malignancy without metastases, the adrenal lesion was benign in 53%, and in patients with extra-adrenal malignancy with metastases, the adrenal lesion was benign in 25% of the cases (Hammarstedt et al. 2012). Thus, an adrenal lesion in a patient with a malignancy should not automatically be taken for a metastasis, especially in a situation where it is the only suspected metastatic site, as the existence of a metastasis may change treatment dramatically.
The second question – does the lesion have benign, indeterminate, or malignant CT characteristics – can ideally be answered already at the time of detection, if the CT examination includes a non-contrast-enhanced series. This is based on the size, morphology, and attenuation measurements of the lesion. It has been shown that adrenal lesions which are homogeneous, well defined with regular outlines, and have a density of 10 HU or less on native images (without contrast medium administration) can confidently be classified as benign (Fig. 10). This density value has also been accepted as a reasonable cutoff in the recently published guidelines from the European Society of Endocrinology (Fassnacht et al. 2016), based on a systematic review and meta-analysis of the literature (Dinnes et al. 2016). Some lesions with ≤10 HU are benign cysts or myelolipomas (Fig. 11), with low density due to their fluid or fatty content, respectively. Myelolipomas are mixed tumors from fatty and myelopoietic cells and are characterized by areas of macroscopic fat, easily identifiable on CT (mean density − 70 HU). They are not hormone producing and therefore usually asymptomatic, unless very big (Lattin et al. 2014). The majority of benign adrenal lesions are, however, adenomas. Most adenomas are rich in intracytoplasmic lipid, which explains the low-density values (≤10 HU). A minority of adenomas are lipid poor, with density measurements >10 HU, partly overlapping with malignant lesions which are also lipid poor. However, malignant lesions often have other characteristics, such as irregular outlines, necrosis, and uneven parenchymal contrast enhancement. Contrast medium washout calculation on CT has been suggested to separate benign from malignant adrenal lesions, when native density measurements are indeterminate, i.e. >10 HU. Absolute washout measurements require that CT scans are obtained before intravenous contrast administration, during the portal phase, and after 10 or 15 min, while relative washout can be calculated on early- and delayed-phase contrast-enhanced images.



Fig. 10
Non-enhanced abdominal CT showed an incidental right-sided, oval-shaped, well-demarcated, homogeneous adrenal mass (arrow), with low density (5–7 HU). This suggests high lipid content characteristic of adrenal adenoma. In the absence of extra-adrenal malignancy, the risk that it is a malignant lesion is very small

Fig. 11
Incidental finding of right adrenal mass with multiple well-defined components of macroscopic fat. The finding is typical for benign adrenal myelolipoma
Using 60–75 s delay for early contrast enhancement scan and 15 min for delayed scan, a washout of 60% or more is a characteristic for benign (adenoma). However, according to a recent meta-analysis, the scientific evidence is not sufficient to motivate washout calculations for regular use for differentiating malignant from benign incidentalomas (Dinnes et al. 2016; Fassnacht et al. 2016).
The third question – is the lesion hyperfunctioning or not – cannot be answered based on its imaging appearance. Each patient with a newly discovered adrenal incidentaloma should be checked for hormonal overproduction of cortisol, aldosterone, or adrenalin/noradrenalin, by deepened clinical history, physical examination, and hormonal laboratory test (Lattin et al. 2014). This is the responsibility of the referring clinician, but the radiologist can point out the need of hormonal testing in his/her report.
7.1 Shape and Size of Adrenals
Identifying adrenal masses may be difficult as the shape and size of the adrenals differ between individuals and between the right and left side within the patient. Vincent et al. (1994) presented CT-based normal values for the size of the adrenal limbs and adrenal body on the right and left side, which may be of some help. The maximum width of the adrenal body was 6.1 mm and 7.9 mm on the right and left side, respectively; the maximum width of the right and left medial limbs were 2.8 mm and 3.3 mm, respectively; and the width of the lateral limb was 2.8 mm and 3.0 mm, respectively. More useful, though, is to look for any localized mass that alters the outline of the adrenal.
The ESE-ENSAT guidelines (Fassnacht et al. 2016) concern only incidentalomas measuring 1 cm or more in size, and workup or follow-up is recommended only if the lesion is 1 cm or more, unless clinical signs and symptoms suggest hormonal overproduction. It is acknowledged that this cutoff is arbitrary, based on the difficulties to confidently identify, measure, and characterize subcentimeter lesions and considering the variations in size and shape of the adrenal. Nevertheless, it should be recognized that even subcentimeter nodules may be hormonally active.
7.2 Management of Adrenal Incidentalomas
Until recently, workup and follow-up of adrenal incidentalomas have been quite extensive, including repeated CT examinations for up to 2 years with and without contrast medium administration to ensure a benign course. With increasing knowledge that adrenal incidentalomas in patients without malignancy very rarely are, or become, malignant, these investigational programs have now been shortened substantially for many patients. For those with indeterminate imaging findings and those with evidence of hormone excess, multidisciplinary expert team meetings are recommended in new guidelines (Fassnacht et al. 2016).
Patients without known extra-adrenal malignancy: non-enhanced CT is recommended for classifying an adrenal lesion as benign or indeterminate. A benign-appearing, well-defined, homogeneous lesion measuring <4 cm and with density ≤10 HU should be considered benign and needs no follow-up. However, evaluation for hormonal excess should be performed. If a similar lesion is 4 cm or larger, it is still likely to be benign, but due to lack of scientific evidence, follow-up with unenhanced CT after 6–12 months for size assessment is recommended. Size (largest diameter) increase of 20% and at least 5 mm is considered suspicious for malignancy and possible indication for surgery.
A patient without known extra-adrenal malignancy and an incidental adrenal mass with indeterminate density characteristics (>10 HU on non-enhanced CT) but otherwise benign appearance, should have non-enhanced CT in 6-12 months for growth assessment. If, on the other hand, the imaging findings do not support a benign etiology (heterogeneous, ill-defined or large lesion), if growth occurs, or if there is hormone overproduction, the patient may be a candidate for surgery. The decision should ideally be taken in a multidisciplinary team, taking clinical circumstances and patient preferences into account (Fassnacht et al 2016). With MRI, the differentiation between benign and malignant lesions is best done using chemical shift technique. Due to its rich lipid content, benign adenomas usually demonstrate a reduction in signal intensity on out-of-phase images, while the signal intensity of lipid-poor adenomas and malignant lesions remains unchanged on in-phase and out-of-phase images. Unlike CT which provides absolute measurements of density, MRI can provide only relative measures of signal intensity. Visual assessment of the MRI signal drop appears to be as useful as these measurements. However, the evidence base for chemical shift evaluation is weak, and CT is recommended as first choice, except in young patients and pregnant women.
7.3 Patients with a History of Extra-Adrenal Malignancy
If the adrenal lesion fulfills the criteria for benign etiology on non-contrast CT, it should be considered benign and requires no follow-up. If the lesion is indeterminate on non-enhanced CT, biopsy, PET-CT, or surgical resection can be considered to rule out metastasis. Regarding biopsy, it must be preceded by hormonal analysis to rule out pheochromocytoma, as the biopsy may release catecholamines causing severe symptoms.
7.4 Young Patients with Adrenal Incidentaloma
In patients under 40 years of age, the likelihood that an adrenal lesion is malignant is higher than in older patients. Therefore, immediate assessment and management rather than 6–12 months follow-up are recommended (Fassnacht et al. 2016).
8 Liver
Simple cysts, hemangiomas, and focal nodular hyperplasia are the most common hepatic lesions detected incidentally. Solid, malignant liver tumors are uncommon as incidental findings in patients without extrahepatic malignancy. In a large CT colonography screening study for colorectal cancer in nearly 8,000 asymptomatic individuals with a mean age of 57 years, unexpected extracolonic findings were analyzed on the unenhanced CT examinations (Pooler et al. 2016a, b). Individuals with extracolonic findings classified on CT colonography as C-RADS category E3 or E4 (Zalis et al. 2005), i.e. likely unimportant but incompletely characterized extracolonic findings (E3) or potentially important extracolonic findings (E4), were followed for 2–10 years. It is notable that all E3 (Pooler et al. 2016a) and E4 (Pooler et al. 2016b) liver masses in patients without known malignancy or cirrhosis were found to be benign liver cysts or cavernous hemangiomas on follow-up. It is thus comforting that incidentally detected isolated liver lesions on CT examinations very rarely seem to represent malignancy, providing that the patient has no known malignant disease or known underlying liver disease. Nevertheless, any solid-appearing liver lesion detected incidentally should be fully characterized by multiphase CT (if not obtained at detection), MRI, or contrast-enhanced ultrasonography. Solid-appearing liver lesions should be clearly highlighted in the radiology report, as underlying malignancy may be unknown to the radiologist. Also, even if benign, adenomas, focal nodular hyperplasia, and other solid liver lesions may be of clinical importance, causing symptoms and requiring intervention in some patients.
8.1 Cystic Lesions
Simple liver cysts are benign lesions without malignant potential and need no follow-up when identified incidentally on abdominal CT examinations. In autopsy studies, liver cysts have been demonstrated in up to half of patients without malignant disease. Benign liver cysts are characterized on CT as other benign, simple cysts, i.e. they are rounded or oval shaped with a thin wall and homogeneous, low density, water-like content (<20 HU) which does not enhance after intravascular contrast medium administration. Cysts that are difficult to characterize on non-enhanced CT are usually easy to confirm on contrast-enhanced CT, unless subcentimeter in size. In doubtful cases, contrast-enhanced ultrasonography, and in particular MRI, may be used for problem-solving. If multiple liver cysts are identified, the kidneys and pancreas should be scrutinized for additional cysts as part of autosomal dominant polycystic kidney disease, which occasionally occurs as an incidental finding in young- or middle-aged patients, although most of such cases are known from family history (Kim et al. 2015).
Any unclear cystic lesion that does not fulfill the CT criteria for a simple cyst, i.e. those that are multilocular or have a thick or irregular wall, septations, solid components, or suspicious contrast enhancement, should be suspected for malignancy and further characterized with ultrasonography or MRI. Such cystic lesions may represent a wide range of etiologies, including biliary cystadenoma or cystadenocarcinoma, cystic degeneration of hepatocellular cancer, and metastasis from ovarian carcinoma and a range of benign disorders, such as biloma, abscess, or echinococcal cysts (Qian et al. 2013). Most of these conditions are, however, unlikely to be incidental findings as they are commonly associated with symptoms. One exception is echinococcal (hydatid) disease, which may be encountered incidentally, as symptoms may develop slowly. Although not encountered commonly as an incidental finding, increasing international migration from endemic areas makes it an important differential diagnosis also in non-endemic countries. Echinococcal disease is caused by the larval stage of the Echinococcus granulosus or multilocularis tapeworm, by ingestion of eggs of the parasite transmitted from animals to humans. Echinococcus disease is endemic in large parts of the world. The ingested eggs release oncospheres which penetrate the gastrointestinal tract to the portal system and invade the liver parenchyma, causing characteristic cystic lesions. These may become symptomatic when large enough to compress the biliary tree or portal vessels, causing jaundice or portal hypertension, or by rupture into surrounding tissues or spaces (Alghofaily et al. 2016). Although the liver is the most common location for echinococcal disease, echinococcal cysts may be seen in virtually any organ. The typical appearance is that of liver cysts containing so-called daughter cysts, i.e. cysts within a mother cyst, sometimes with wall enhancement. The cyst walls, and detached floating membranes, may give the impression of septations. Commonly, characteristic calcifications of the cyst walls occur (Marrone et al. 2012).
8.2 Hemangioma
Hemangiomas are the most common non-cystic focal liver lesions, occurring in about 20% in autopsy series. As these lesions are mostly asymptomatic, it is a common incidental liver finding. The reported frequency of hemangiomas may be higher on MRI (7%) than on CT, where the prevalence on abdominal CT was 2.4% in a recent retrospective analysis of 70,000 abdominal CT examinations (85% incidental) (Mocchegiani et al. 2016). These are minimum figures, considering the retrospective design of the study. On non-enhanced CT, the most common type of hemangioma, the cavernous hemangioma, has attenuation similar to that of other vascular structures and may therefore be difficult to characterize. After intravenous contrast medium injection, hemangiomas appear well defined, with nodular, peripheral-enhanced vascular structures becoming apparent, surrounding the low-attenuating center, followed by gradual centripetal contrast medium fill-in, which typically will be noted over several minutes until more or less complete fill-in will occur (Fig. 12). In most cases, hemangiomas can be confidently diagnosed on contrast-enhanced CT. Normally, hemangiomas are asymptomatic and require no further follow-up (Marrero et al. 2014). However, if large (>4 cm), there is a risk, albeit small, of spontaneous rupture that may motivate follow-up and possible intervention (Mocchegiani et al. 2016). Considering that rupture occurred mainly in large lesions with a peripheral location, the size and location of the hemangioma should be clearly stated in the radiology report. If an hemangioma is incidentally suspected on non-enhanced CT, the lesion, like other lesions that do not fulfill the criteria for simple cysts, should be further characterized by contrast-enhanced CT or MRI, if necessary including delayed imaging to confirm a hemangioma. Heavily T2-weighetd MRI is particularly effective to differentiate hemangioma from a malignant lesion (McFarland et al. 1994). As an alternative, contrast-enhanced ultrasound may be used, providing that a trained examiner is available (D’Onofrio et al. 2015).


Fig. 12
Incidental detection of a low density liver lesion with nodular peripheral contrast enhancement (arrows) on early phase contrast-enhanced CT. The finding is highly suggestive of hemangioma, which can be confirmed by progressive centripetal contrast fill-in on a later phase imaging
8.3 Non-cystic Benign Liver Lesions
After hemangioma, focal nodular hyperplasia (FNH) is the second most common benign liver tumor. Although it occurs also in males, it is much more common in women, in whom it commonly presents in the third or fourth decade of life. In 85% of the cases, the lesion is less than 5 cm in size at detection. It is usually asymptomatic, and therefore most lesions are detected incidentally on cross-sectional imaging, including abdominal CT. However, with increasing size, it may cause pain, discomfort, or a palpable mass. Rarely, several FNH lesions may coexist. The appearance on CT is that of a slightly lobulated soft tissue mass, which is iso- or hypoattenuating as compared to the surrounding parenchyma on non-enhanced CT. In the arterial post-contrast phase, the lesion is typically homogeneously hyperattenuating as compared to the liver parenchyma, with a central “scar” of less enhancement. In the portal phase and later, the FNH is more or less isoattenuating with the parenchyma (Fig. 13), while the central scar often shows gradual enhancement on later phases (Hussain et al. 2004). In rare cases, the central scar remains hypoattenuating after intravenous contrast administration, making distinction from fibrolamellar hepatocellular carcinoma with central necrosis difficult. In some cases (16–40%), the central scar is small or not clearly recognizable on CT, making the diagnosis less specific (Mortele et al. 2000). In such cases, MRI may be helpful to establish the diagnosis (Hussain et al. 2004).


Fig. 13
A 32-year-old, previously healthy female with acute lower abdominal pain admitted for acute abdominal CT, which showed acute appendicitis. As incidental finding, a 7 × 6 cm solid, slightly lobulated lesion of the left lobe of the liver was found. The lesion appeared isoattenuating with the liver in the portal phase (arrows) and showed a central scar suggestive of, but not proving, focal nodular hyperplasia (FNH). It could not be confidently classified on single-phase CT, but FNH was confirmed by subsequent liver MRI
Hepatic adenomas are less common than cysts, hemangiomas, and FNH. As with FNH, they are more common in women of childbearing age, but a stronger association with oral contraception medication has been shown for adenomas, in addition to a strong association with steroid (mis-)use. There is also a long-term increased risk of malignancy, not seen with FNH. A hepatic adenoma may cause symptoms, such as pain, discomfort, or other symptoms related to a mass effect, but symptoms may also be more acute, related to rupture and bleeding. With increased use of abdominal CT, an increasing proportion of hepatic adenomas are identified as incidental findings on CT. Their detection and differentiation from FNH (and hepatocellular carcinoma) are important, as hepatic adenomas may be candidates for more intense follow-up or surgical removal, which is not usually the case for FNH.
Apart from occasional bleeding, some adenomas develop necrosis, recognizable on imaging examinations. In 5–10% of cases, calcifications may be seen on CT. Hepatic adenomas usually occur as single lesions, mostly in the right lobe of the liver but may be multiple. They are usually well circumscribed, non-lobulated, and isoattenuating with the liver parenchyma before contrast enhancement. Due to varying elements of intra-tumoral fat and post-hemorrhage tissue reactions, they may appear irregularly hypo- or hyperdense. In case of liver steatosis, they may occur as hyperdense in comparison with the liver. After intravenous contrast administration, small adenomas tend to be hyperattenuating on imaging in the arterial phase and isoattenuating in the portal phase (Grazioli et al. 2001). Unlike FNH, there is no central scar in adenomas, unless mimicked by central necrosis. Overlapping CT imaging features between hepatocellular carcinoma, FNH, and adenoma makes characterization at incidental detection on CT difficult. In the clinical situation, this is not trivial, and, therefore, a combination of multiphase CT and MRI is often necessary to obtain a final diagnosis (Grazioli et al. 2005).
8.4 Approach to an Incidental Liver Mass Detected on CT
Many liver lesions detected incidentally on abdominal CT are small and of uncertain clinical importance. An isolated 8 mm liver lesion of unclear etiology in an 85-year-old patient without known malignancy is probably of very minor clinical importance, while a similar finding in a 30-year-old male body builder using anabolic steroids may be of potential clinical importance, requiring follow-up. Both lesion size and patient background factors, as well as comorbidity and life expectancy, clearly have to be taken into consideration when evaluating incidentally detected liver lesions. The American College of Radiologists (ACR) Incidental Findings Committee has published guidelines regarding the management of incidental liver masses (Berland et al. 2010). They suggest that patients with incidental liver lesions be categorized according to risk status, into those with low, average, or high risk: Low risk individuals are defined as “young patients (≤40 years old), with no known malignancy, hepatic dysfunction, hepatic malignant risk factors or symptoms attributable to the liver.” Average risk individuals are defined as those “>40 years old, with no known malignancy, hepatic dysfunction, abnormal liver function tests or hepatic malignant risk factors or symptoms attributable to the liver”. High risk individuals are defined as those “with known primary malignancy with a propensity to metastasize to the liver, cirrhosis, and/or other hepatic risk factors. Hepatic risk factors include hepatitis, chronic active hepatitis, sclerosing cholangitis, primary biliary cirrhosis, hemochromatosis, hemosiderosis, oral contraceptive use, anabolic steroid use” (Berland et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree