Chapter Outline
Bacterial (Pyogenic) Hepatic Abscesses
Candidiasis and Fungal Infections
Pneumocystis carinii ( Pneumocystis jiroveci ) Infection
Technologic advances have significantly enhanced the role of radiology in the detection, characterization, and management of focal infectious diseases of the liver. Today, all cross-sectional techniques allow highly accurate detection of focal hepatic infections. Computed tomography (CT) is particularly helpful in revealing the presence of calcifications and gas and in detailing the enhancement pattern. With its multiplanar capacity and sensitivity to small differences in tissue composition, magnetic resonance imaging (MRI) is a useful tool to diagnose and to characterize lesions such as hepatic abscess, hydatid cyst, and candidiasis. The impact of imaging is particularly dramatic for pyogenic abscess; early diagnosis and imaging-guided percutaneous drainage have markedly reduced both the mortality rates (from 40% to 2% of cases) and the need for surgery. This chapter is a review of the radiologic and pathologic findings in a variety of focal hepatic infections, including abscesses, parasitic diseases, and fungal diseases.
Bacterial (Pyogenic) Hepatic Abscesses
Incidence
Pyogenic liver abscesses are uncommon in Western countries, accounting for 0.1% of hospital admissions and having a prevalence at autopsy series of nearly 1%. There is a slight female predominance, and individuals between 40 and 60 years of age are most often affected.
Pathogenesis
Hepatic abscess can develop by five major routes: biliary—ascending cholangitis from benign or malignant biliary obstruction, choledocholithiasis; portal vein—pylephlebitis from appendicitis, diverticulitis ( Fig. 88-1A ), necrotic colon cancer, inflammatory bowel disease, proctitis, infected hemorrhoids, pancreatitis; hepatic artery—septicemia from bacterial endocarditis, pneumonitis, osteomyelitis; direct extension from contiguous organs—perforated gastric or duodenal ulcer, lobar pneumonia, pyelonephritis, subphrenic abscess; and traumatic, from blunt or penetrating injuries. Metastatic tumor nodules can also become abscesses.

Before the antibiotic era, pylephlebitis of the portal vein from seeding by appendicitis and diverticulitis was the most common cause of hepatic abscess. Indeed, appendicitis, which was once responsible for 34% of all pyogenic abscesses, now accounts for less than 2%. Biliary tract disease is now the most common source of pyogenic liver abscess. Obstruction of bile flow allows bacterial proliferation. Through pressurization and distention of canaliculi, portal tributaries and lymphatics are invaded, with subsequent pylephlebitic abscess formation. Cholecystitis, stricture (benign or malignant), malignant neoplasms, and congenital diseases are common inciting conditions. Approximately 50% of pyogenic abscesses are caused by an anaerobic organism, mixed anaerobic organisms, or mixed anaerobic and aerobic organisms. Facultative gram-negative enteric bacilli, anaerobic gram-negative bacilli, and microaerophilic streptococci are the organisms most often responsible for liver abscesses. Escherichia coli is the organism most commonly isolated in culture in adults ( Fig. 88-1B ). Staphylococci organisms are most often isolated from hepatic abscesses in children.
Pathology
Abscesses of biliary tract origin are multiple and in 90% of cases involve both hepatic lobes. Abscesses of portal origin are usually solitary; 65% occur in the right lobe, 12% occur in the left lobe, and 23% are bilateral. This distribution is explained by the streaming effect of mesenteric blood flow in the portal vein.
Clinical Findings
The high morbidity and mortality of hepatic abscesses (50%-70%) before the era of cross-sectional imaging attest to the difficulty of establishing a diagnosis of pyogenic liver abscess on clinical grounds alone. The most common symptoms are fever, malaise, pain, rigors, nausea and vomiting, and weight loss. Tender hepatomegaly is the most common clinical sign, and leukocytosis, elevated serum alkaline phosphatase levels, hypoalbuminemia, and prolonged prothrombin time are the most common laboratory abnormalities. Clearly, these findings are nonspecific, and cross-sectional imaging has proved vital in the prompt diagnosis and management of hepatic abscess, resulting in improved survival.
Radiologic Findings
Cholangiography
Ascending cholangitis is the most common cause of pyogenic hepatic abscess, and cholangiography has become an important aid to diagnosis in many cases. Percutaneous transhepatic cholangiography and endoscopic retrograde cholangiography can accurately define the level and cause of biliary obstruction; they are a first step in biliary drainage procedures and can accurately define biliary anatomy for the surgeon. These procedures increase intrabiliary pressure and can precipitate deterioration of a patient who is already septic. Accordingly, biliary drainage procedures should be anticipated. Magnetic resonance cholangiopancreatography is an important tool in diagnosis of obstructive biliary tract lesions.
Ultrasound
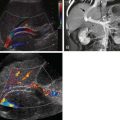
Real-time ultrasound can detect hepatic abscesses as small as 1.5 cm with a sensitivity of 75% to 90%. Pyogenic hepatic abscesses are extremely variable in shape and echogenicity. They are usually spherical ( Fig. 88-2 ) or ovoid but may be lobulated or lentiform. Mural thickness is variable, and the wall typically is irregular and hypoechoic. On sonography, abscesses appear anechoic (50%), hyperechoic (25%), or hypoechoic (25%). Septa, fluid-fluid levels, internal debris, and posterior acoustic enhancement may also be seen. Early lesions tend to be echogenic and poorly demarcated; they may evolve into well-demarcated, nearly anechoic lesions. If gas is present in an abscess, brightly echogenic reflectors with posterior reverberation artifact may be noted.

The sonographic differential diagnosis includes amebic or hydatid infection, necrotic or cystic neoplasm, hematoma, complicated biloma, and simple cyst with infection.
Nuclear Scintigraphy
Pyogenic liver abscesses appear as rounded, cold areas on technetium Tc 99m sulfur colloid and hepatobiliary scintiscans. On occasion, communication between the abscess cavity and the biliary system can be demonstrated on the 99m Tc-sulfur colloid study.
Gallium citrate Ga 67 scintigraphy and imaging with leukocytes labeled with indium In 111 are the two nuclear medicine techniques that were used for the detection of pyogenic abscess in the past but are abandoned today.
Magnetic Resonance Imaging
Hepatic abscesses, like most other focal hepatic processes, prolong T1 and T2 relaxation times. At MRI, air within the abscess appears as a signal void and is therefore more difficult to differentiate from calcifications. However, the shape and location (air-fluid level) should enable the correct diagnosis. After administration of gadopentetate dimeglumine (Gd-DTPA), abscesses typically show rim enhancement ( Fig. 88-3 ), which is secondary to increased capillary permeability in the surrounding liver parenchyma (the double target sign). Small lesions (<1 cm) may enhance homogeneously, mimicking hemangiomas. Abscess wall enhancement on dynamic postgadolinium images may be considered a distinctive feature of pyogenic liver abscesses. Abscess wall shows a fast and intense enhancement that persists on portal venous and late-phase images. Some of the lesions may contain internal septations, which also reveal persistent enhancement on late-phase images. Perilesional edema, shown as high signal on T2-weighted images, is associated with 50% of abscesses. However, it may also be seen in 20% to 30% of patients with primary or secondary hepatic malignant neoplasms. Therefore, the presence of perilesional edema can be used only to differentiate a hepatic abscess from a benign cystic hepatic lesion. Resolution of perilesional edema may indicate response to therapy. Limitations of MRI in the investigation of patients with abscesses include the relatively high cost and the lack of easy access for drainage procedures.

Computed Tomography
By virtue of its good spatial and contrast resolution, CT is the single best method for detecting hepatic abscess, with a sensitivity as high as 97% ( Fig. 88-4 ). On CT scans, abscesses appear as generally rounded masses that are hypodense on both contrast and noncontrast scans. The attenuation ranges between 0 and 45 HU and thus overlaps with the appearance of cysts, bilomas, and hypodense neoplasms. Most have a peripheral rim or capsule that shows contrast enhancement in a pattern similar to that seen on MRI (see Fig. 88-2 ). Most abscesses are sharply defined, but a minority have a grossly lobular contour and circumferential “transition zones” of intermediate attenuation.

Another helpful finding is the transient segmental or wedge-shaped enhancement of the hepatic parenchyma surrounding an abscess on the arterial dominant phase of a contrast-enhanced dynamic CT scan.
Some abscesses show the cluster sign (see Fig. 88-4C -E), in which small, pyogenic abscesses appear to cluster or aggregate in a pattern suggesting coalescence into a single large cavity.
All of these findings are nonspecific and require aspiration for diagnosis. Central gas (see Fig. 88-4A ), as either air bubbles or an air-fluid level, is a specific sign, but it is present in less than 20% of cases. A large air-fluid or fluid-debris level is often associated with communication with the gut.
Treatment
Effective treatment of pyogenic liver abscess entails elimination of both the abscess and its underlying source. Current therapeutic options include surgical drainage, antibiotics alone, percutaneous aspiration in conjunction with antibiotics, and percutaneous catheter drainage.
As a rule, aspiration alone is sufficient if the fluid collection is unilocular, well demarcated, and less than 5 cm in diameter and shows no communication with the gut or biliary tree. Unless the abscess is chronic and the cavity walls are fibrotic or calcified, small intrahepatic abscess cavities quickly collapse when aspirated, unlike abscesses in other organs and peritoneal spaces. In a retrospective study of 115 patients, excellent results were obtained with ultrasound-guided percutaneous needle aspiration of pyogenic abscesses followed by injection of antibiotics into the abscess cavity. Cure was achieved in 98% of cases, with no deaths, complications, or recurrences at 3-year follow-up. Fluid drainage usually requires introduction of a 16- or 18-gauge Teflon-sheathed needle into the collection and, if the fluid is extremely viscous, temporary insertion of a 5F to 8F pigtail catheter. All fluid is aspirated, and the cavity is irrigated with normal saline or an antiseptic solution.
Before removal of the catheter, the collection is injected with contrast medium to rule out communication with surrounding organs. Intravenous antibiotics are given before, during, and after the procedure and are changed accordingly when the infecting organism is identified.
An indwelling drainage catheter is usually required when the fluid collection is ill-defined, multiloculated, or more than 5 cm in diameter or when communication with the gut or biliary tree is suspected. A variety of drainage catheter styles (single lumen, double lumen, sump type) and sizes (8.3F-16F) are available. Most are made of a flexible Silastic material with a pigtail curve containing multiple side holes. Smaller catheters (8.3F-10F) are usually sufficient to adequately drain thin fluid collections or extremely small abscesses; large abscesses require larger catheters (12F-16F), especially if they contain particulate material. The catheter can be inserted under CT, ultrasound, or fluoroscopic guidance by the Seldinger or trocar technique. Other chapters contain complete discussions of imaging guidance and abscess catheterization. The catheter is left in place until drainage volume has decreased to less than 20 mL/day. If there is a known or suspected fistula, a fluoroscopic sinogram is obtained before catheter removal to rule out communication with bowel, bile duct, or pancreatic duct. If no fistula is present, most hepatic abscesses require only 2 to 14 days of drainage.
The failure and recurrence rates of catheter therapy are 8.4% and 8%, respectively. They are most often associated with complicated abscesses caused by fistula, phlegmons, or infected tumors.
Surgery is reserved for patients for whom percutaneous drainage has failed, those with associated intra-abdominal perforation of hepatic abscess, and those with fistula formation (e.g., biliary, colonic).
The mortality rate for pyogenic hepatic abscess has declined from 80% to less than 10% owing to earlier diagnosis, antibiotics, and advances in surgical and percutaneous drainage techniques.
Amebic Abscesses
Incidence
Approximately 10% of the world’s population is infected with Entamoeba histolytica , which causes more deaths than any other parasite with the exceptions of malaria-causing plasmodia and schistosomes. Less than 10% of infected individuals, however, are symptomatic. Amebic liver abscess is the most common extraintestinal manifestation, occurring in 3% to 7% of this population.
Although amebiasis is usually considered a disease of developing countries, certain groups are at high risk in Western nations: recent immigrants, institutionalized patients, and homosexual persons. Indeed, E. histolytica has been isolated in the stool of up to 30% of sexually active homosexual men; its clinical significance is unclear. Worldwide, some 85% to 90% of amebic liver abscesses occur in men.
In the United States, the overall mortality rate for hepatic amebic abscess is 3%. It is less than 1% when the abscess is confined to the liver, 6% with extension into the chest, and 30% with extension into the pericardium.
Pathogenesis
The cystic form of E. histolytica gains access to the body by oral ingestion of infected material, usually contaminated water ( Fig. 88-5A ). The mature cysts are resistant to gastric acid and pass unchanged into the intestine. The cyst wall is then digested by trypsin; four invasive trophozoites are released, which live and multiply in the colon, particularly the cecum. The trophozoites exist in two forms, small (10-20 mm) and large (20-60 mm). The large form usually occurs in invasive amebiasis when the mucosa is invaded. This can cause minute superficial mucosal ulcerations. With further invasion, hemorrhage, perforation, enterocolic or cutaneous fistulas, amebic appendicitis, or ameboma formation can occur. Amebic trophozoites can also enter the mesenteric venules and lymphatics and be carried to the liver, lungs, and other organs. The liver can be invaded in one of three ways: through the portal vein (most common); through lymphatics; or by direct extension through the colon wall into the peritoneum and then through the liver capsule.


When a sufficient number of trophozoites become lodged in small hepatic venules, thrombosis and infarction of small areas of hepatic parenchyma occur (amebic hepatitis). The host’s nutritional and immune status determine whether the initial infestation heals or progresses to a macroabscess. A visible abscess results from the coalition of multiple small areas of ischemic necrosis and amebic destruction of hepatic parenchymal cells.
Trophozoites that pass into the distal colon may change into round, resistant cysts ( Fig. 88-5B ) that pass into the feces. Indeed, human carriers who pass amebic cysts into their stool are the primary source of infection.
Pathology
The fluid of an amebic abscess is usually dark reddish brown and has the consistency of anchovy paste ( Fig. 88-5C ). This material is usually sterile, consisting of a mixture of blood and destroyed hepatocytes. Rarely, the trophozoites are found centrally within the paste, but they are often found in the zone of necrotic tissue adjacent to the outer abscess wall. The wall of connective tissue becomes better developed with age, and leukocyte infiltration and inflammatory reaction are characteristically absent. If the abscess is not treated, it may rupture into the peritoneum, pleural cavity, lung, or pericardium.
Amebic abscesses are most often solitary (85%) and affect the right lobe more often (72%) than the left lobe (13%). Solitary liver abscesses and right lobe predominance are more marked in amebic than in pyogenic abscesses because most infestations are transmitted through the portal vein. Amebiasis most often affects the right colon, which drains into the superior mesenteric vein, which preferentially streams into the right lobe. Flow from the inferior mesenteric vein (left colon) and splenic vein streams preferentially into the left lobe.
Clinical Findings
Most patients with amebic liver abscess present with a tender liver and right upper quadrant pain. Compared with persons who have pyogenic liver abscess, they are more likely to have diarrhea and hepatomegaly and less likely to have jaundice or sepsis. Amebae are not found in the stool of most patients with an amebic liver abscess.
Because clinical features and findings of stool examination for amebae usually are nonspecific or negative, serologic tests are particularly helpful when hepatic amebic abscess is suspected. The indirect hemagglutination test result is positive in more than 90% of these patients.
Radiologic Findings
Nuclear Scintigraphy
Amebic abscesses appear as cold defects on sulfur colloid scans. They often show “rim enhancement” on hepatobiliary scintiscans, presumably owing to inflammation of the adjacent parenchyma. A cold lesion with a hot periphery is suggestive of the diagnosis. Nevertheless, nuclear medicine techniques are not routinely used for detection of hepatic amebic abscess.
Ultrasound
Amebic abscess on ultrasound studies is usually a round or oval, sharply defined hypoechoic mass ( Fig. 88-6 ) that abuts the liver capsule with homogeneous, fine, low-level echoes and distal acoustic enhancement. In comparison of amebic and pyogenic abscesses, amebic abscess is more likely to have a round or oval shape (82% vs. 60%) and a hypoechoic appearance with fine, low-level internal echoes at high gain settings (58% vs. 36%). On contrast-enhanced ultrasonography, pyogenic abscess appears as a partially enhancing lesion with a thin or thick rim of dense opacification and persistently hypoechoic center.

Computed Tomography
The CT appearance of amebic abscess is variable and nonspecific. The lesions are usually peripheral, round or oval areas of low attenuation (10-20 HU). A peripheral rim of slightly higher attenuation can be seen on noncontrast scans and shows marked enhancement after administration of contrast material ( Fig. 88-7 ). A peripheral zone of edema around the abscess is also common and somewhat characteristic for this lesion. Lesions may appear unilocular or multilocular and demonstrate nodularity of the margins. Concomitant extrahepatic abnormalities include right-sided pleural effusion, perihepatic fluid, and gastric or colonic involvement.

Magnetic Resonance Imaging
On MRI ( Fig. 88-8 ), amebic liver abscesses are spherical and usually solitary lesions with a hyperintense center on T2-weighted images and a hypointense center on T1-weighted images. The abscess wall is thick, and on gadolinium-enhanced images, the enhancement pattern is similar to that of pyogenic abscess. Diffuse central inhomogeneity is often seen on T2-weighted images. Edema in otherwise normal surrounding parenchyma may be appreciated on T2-weighted images.

After treatment, abscesses become more homogeneously hypointense on T2-weighted images. Successful treatment may show concentric rings of different signal intensity surrounding the lesion on T2-weighted images.
Complications
Pleuropulmonary amebiasis is the most frequent complication of amebic liver abscess, occurring in 20% to 35% of patients. This may be manifested as pulmonary consolidation or abscess, serous effusion, empyema, or hepatobronchial fistula.
Peritoneal amebiasis occurs in 2% to 7.5% of patients with amebic liver abscess. Sudden rupture is manifested dramatically, in the manner of a perforated viscus. Pericardial amebiasis is the most serious complication of amebic liver abscess because it can lead to progressive tamponade or sudden development of shock. Most abscesses responsible for this complication are located in the left hepatic lobe. Renal amebiasis is a rare complication that can result from abscess rupture.
Treatment
The complications of amebic liver abscess just mentioned are becoming less frequent because of earlier diagnosis, improved imaging and serologic techniques, and effective medical, percutaneous, and surgical management. More than 90% of hepatic amebic abscesses respond to antimicrobial (metronidazole or chloroquine) therapy alone. The efficacy of these amebicidal agents has reduced the mortality rate of hepatic amebic abscesses from 81% to 4%.
There are, nevertheless, some circumstances in which aspiration and drainage of amebic abscess are indicated: to differentiate pyogenic from amebic abscess; for a large symptomatic abscess in which rupture is imminent; after poor response to medical therapy; with suspected bacterial superinfection; in pregnancy; for noncompliance with medical treatment; and as an alternative to surgery when an abscess ruptures. Drainage can be accompanied by intralesional delivery of drug, which can increase mean intralesional drug levels up to 246-fold.
Confirmation of an amebic abscess may not always be possible by percutaneous aspiration. In one series, only 50% of patients had the classic “anchovy paste” appearance, and positive diagnoses based on fluid evaluation were established in only 5% of cases. Therefore, the primary role of percutaneous aspiration and drainage in these patients is the diagnosis and treatment of superimposed bacterial infection.
Hepatic Echinococcal Disease
Epidemiology
Hydatid disease is prevalent throughout much of the world, and the two main forms that affect humans are Echinococcus granulosus and Echinococcus multilocularis . The disease flourishes in rural areas where dogs are used for herding livestock, especially sheep. Greece, Uruguay, Argentina, Australia, and New Zealand are the countries with the highest incidence of hydatid disease.
Pathophysiology
E. granulosus is a small tapeworm, 3 to 6 mm long, that lives in the intestine of the definitive host, usually the dog. The life cycle ( Fig. 88-9A ) depends on a primary host harboring the adult worm and an intermediate (human) host harboring the larval stage. The adult parasite sheds eggs, which may infect humans when contaminated, unwashed vegetables are ingested or through contact with infected dogs, which may carry ova on their fur or shed them onto soil where children play.


In humans, the external shell of the egg is digested in the duodenum and the embryo is freed. The embryo actively penetrates the intestinal mucosa until it enters a blood vessel, from which it is transported until trapped by narrowing of the capillaries. Most are carried by the portal vein and trapped in the liver, but the lungs, spleen, kidneys, bone, and central nervous system can also be involved.
Although most embryos are destroyed by host defenses, surviving embryos ( Fig. 88-9B ) develop into the hydatid stage in 4 to 5 days while trapped within a capillary. After 3 months, this cyst measures 5 mm in diameter. The life cycle is complete when the infected intermediate host (sheep or other ruminant) dies and its viscera, which contain the larval form, are consumed by a definitive host.
E. multilocularis (alveolaris) is a less common but more aggressive form of echinococcus. The disease is endemic in central Europe, the former Soviet Union, Japan, and central and northern North America. The main host of the adult parasite is the fox, although less commonly domestic dogs and cats may serve as hosts. The intermediate hosts, usually wild rodents, acquire infection by eating contaminated wild berries. Humans become infected either by eating wild fruits contaminated with fox feces or by direct contact with infected animals. The larvae reach the liver through the portal vein. Here they proliferate and penetrate surrounding tissue, which causes a diffuse and infiltrative process that simulates a malignant neoplasm. E. multilocularis organisms induce a brisk granulomatous reaction with central necrosis, cavitation, and calcification.
Pathology
The hydatid cyst has three layers composed of both host and parasite tissue ( Fig. 88-9C ). The outer pericyst is composed of modified host tissue that forms a rigid protective zone only several millimeters thick. As the cyst expands, the vessels and ductal structures of the liver become incorporated in the cyst wall; this explains enhancement of the wall on contrast CT and MR studies and angiography.
The two internal layers are formed by the parasite. The middle layer is a thicker (1-2 mm) laminated membrane that resembles the white of a hard-boiled egg and is easily ruptured with manipulation. It permits the passage of nutrients but is impervious to bacteria. Disruption of this layer predisposes to bacterial infection. The innermost or germinal layer (endocyst) is the living parasite, which is one cell thick. It produces the laminated membrane and scolices that represent the larval stage. Brood capsules are small spheres of disrupted germinal membrane that produce scolices ( Fig. 88-9D ). Free-floating brood capsules and scolices form a white sediment of barely visible particles known as hydatid sand.
Cyst fluid is secreted by the germinal lining and normally is crystal clear. It is a transudate of serum that contains protein and is antigenic. The high secretion pressure is responsible for progressive cyst enlargement. Up to 60% of cysts are multiple.
In contrast to E. granulosus , the alveolar form has daughter cysts that arise on the outer surface of the original cyst with invasion of adjacent liver parenchyma. On histologic examination, the cysts have a thick lamellated wall. Surrounding this is the marked granulomatous reaction with hepatic necrosis, collagenous tissue, multinucleated giant cells, and lymphocytes. In contrast to the cysts of E. granulosus , the E. alveolaris cysts rarely contain scolices in human infestation.
Clinical Findings
Most patients acquire hydatid disease in childhood but are not diagnosed until the third or fourth decade of life. Echinococcal cysts enlarge at a rate of approximately 1 cm/year. Most cysts are initially asymptomatic and remain so until they grow large enough to cause pain; erode into a moderate-sized bile duct, allowing cyst fluid to enter the bile (and vice versa), causing fever and jaundice; or induce an allergic reaction because of leakage of cyst fluid. Large cysts can obstruct blood and bile flow, leading to portal hypertension and jaundice.
Routine blood analysis is usually not helpful in establishing the diagnosis of hydatid disease. Results of serologic tests are positive in more than 80% of cases and are diagnostic of echinococcoses. The clinical manifestations of E. multilocularis may occur 5 to 20 years after the inciting event. Abdominal discomfort, jaundice, and hepatomegaly may be present, and eosinophilia is frequently observed. Although the serum levels of the transaminases usually remain normal, alkaline phosphatase and γ-glutamyl transpeptidase values are elevated. Serologic titers are elevated in alveolar echinococcosis and aid in diagnosis.
Radiologic Findings
Plain Radiographic Findings
Calcification is visible on 20% to 30% of abdominal plain radiographs ( Fig. 88-10 ). The calcification is usually curvilinear or ringlike and lies in the pericyst. Daughter cysts may calcify, creating rings of calcification. Calcification does not always indicate death of the parasite, and small irregular areas of calcification may be secondary to dystrophic calcification in old blood clots. E. multilocularis can cause faint or dense punctate calcification scattered throughout necrotic and granulomatous tissue.