Fig. 1.1
AUA guidelines flow chart
For index patient 1, the AUA guidelines state that the primary standard is partial nephrectomy with radical nephrectomy an alternate standard when partial nephrectomy is not feasible. In regards to surgical approach, the panel suggested that open partial nephrectomies should be performed with the laparoscopic approach reserved for less complicated lesions. Additionally, open partial nephrectomy is preferable to laparoscopic radical nephrectomy. The clear focus for index patient 1 is surgical extirpation as there are no recommendation level strategies listed. Instead, the panel lists TA as options in this population. The major concerns for the use of TA are the higher recurrence rates, especially in light of what could be a difficult surgical salvage, and the nonstandard definition of success that makes literature interpretation difficult.
For index patient 2, both partial and radical nephrectomies are standards held in equal regard. The panel concluded that due to the patient’s competing comorbidities, partial nephrectomy assumes considerable risk and radical nephrectomy may be safer despite the long-term sequela of renal insufficiency. In this population, both TA and AS are recommendation level strategies, also held in equal regard. The minimally invasive approach of TA and the forgoing of active therapy altogether with AS are attractive alternatives to extirpation in this population of unhealthy patients.
For index patient 3, the panel lists radical nephrectomy as the primary standard with partial nephrectomy an alternate standard. There are no recommendation level strategies with the focus being on surgical extirpation. Both AS and TA are listed as options; however, the AUA specifically noted that they are both suboptimal treatments in this population. The major concerns cited by the guidelines panel is the high complication and recurrence rates associated with large tumor TA and the increased metastatic potential and disease progression associated with AS of larger lesions in an otherwise healthy population.
Finally, for index patient 4, RN is the only listed standard. Due to the significant risk incurred with PN in this population, its role was reduced to a recommendation. AS is listed as an alternate recommendation in those patients who wish to avoid or may not tolerate surgery. TA maintains its position as an option due to concerns about high recurrence and complication rates.
The intent of the AUA guidelines panel was to create an evidence-based algorithm that helps navigate the difficult and continuously evolving small renal mass dilemma. While recognizing that intent, the authors’ experience has led them to slightly different conclusions than that of the panel. For index patient 1, the panel focused solely on surgical extirpation by any means necessary, that is, RN should PN not be feasible. However, we contend that the goal for the healthy T1a patient should focus on sparing nephrons by any means necessary, especially when considering that these patients likely have a long life expectancy. PN should retain its position as the gold standard treatment. However, if partial nephrectomy is not feasible, in lieu of radical nephrectomy, which renders the patient at risk for lifelong renal insufficiency, early cardiovascular events, and decreased overall survival, TA should be performed [5–8]. RN in the PN failure group is more in line with the old adage of immediate RN for small masses, while TA in this group reflects not only newer TA success data but also the AS data that suggest that small tumors grow slowly and rarely progress. Additionally, local recurrences may not necessitate surgical extirpation, but can be easily treated with a repeat ablation. Unfortunately, the AUA index patient 2 is an oversimplification of a wide spectrum of patients. We contend that this group can be divided into 2 subsets based on comorbidity index, which ultimately affects primary and secondary treatments. Patients with a comorbidity index <3 could proceed as the panel suggests. However, in patients with a comorbidity index of ≥3, PN and RN should be used sparingly. Instead, AS should be offered as the primary treatment strategy with TA in those patients that fail AS. This strategy applies the emerging data from AS as well as the minimalistic approach of TA in this unhealthy population. Finally, in our experience, TA is a poor treatment modality for patients with lesions >3 cm. TA in large tumors is technically difficult, and as a result, there are higher complication rates and inadequate efficacy. Therefore, for all patients with T1b lesions (index patients 3 and 4), TA should not be offered.
Surgical Approach
CA and RFA are the most popular and best characterized TA modalities. Both can be pursued laparoscopically or percutaneously with the approach largely dependent on the location of the renal mass (Fig. 1.2). Lesions located on the anterior aspect of the kidney are more suitably approached transperitoneally (laparoscopically) while posteriorly located tumors are best approached either percutaneously (CT or MRI guided) or via a retroperitoneal laparoscopic technique. Laterally located tumors present a small challenge due to their inconvenient location with the approach being based on surgeon preference. The majority of RFA is performed percutaneously, while CA has been well described both laparoscopically (trans- and retroperitoneally) and percutaneously.
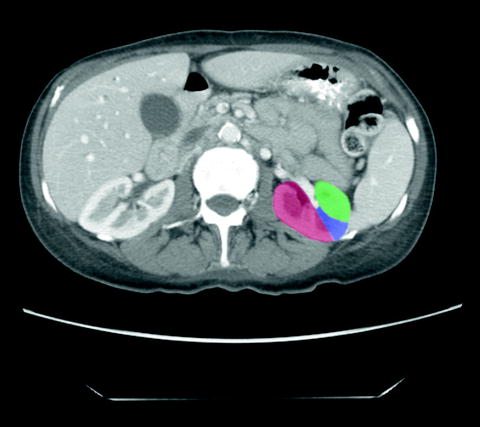

Fig. 1.2
Tumors located on the posterior aspect of the kidney (red) are ideally approached either percutaneously or via retroperitoneal laparoscopy. Tumors located on the anterior aspect of the kidney (green) are ideally approached by transperitoneal laparoscopy. Tumors located on the lateral aspect of the kidney (blue) can be approached by any technique
Patient Preparation
Preoperatively, patients should undergo a complete history and physical examination that includes a complete set of vitals, careful review of the past medical and surgical history, social history including smoking history, and a review of the medications. Laboratory examination should include a complete metabolic panel, cell blood count, and when appropriate a coagulation panel. All patients over the age of 40 should undergo a preoperative electrocardiogram and a chest X-ray. Elevated liver enzymes are suggestive of either a Stauffer’s syndrome or perhaps more ominous, metastasis to the liver. Careful reevaluation of the liver with axial imaging is warranted. Abnormal neurological findings or recent onset of headaches or blurred vision should prompt the surgeon to investigate the possibility of brain metastasis with a head CT or MRI. Similarly, complaints of bony pain, especially with concomitant elevations in serum alkaline phosphatase and/or calcium, could be indicative of bony metastasis, which should be evaluated with a nuclear bone scan. Finally, anticoagulants, including aspirin products, should be stopped 7–10 days prior to surgery. In toto, the goal of this extensive preoperative routine is to identify potential problems that may affect surgical outcome. For example, vital signs may identify poorly controlled or previously unidentified hypertension, which puts the patient at risk for intra- and postoperative bleeding, or anticoagulant use which also increase bleeding diathesis. Additionally, it is this preoperative work-up that stratifies the individual patient into the various management strategies mentioned above.
Recent high-quality axial imaging via computed tomography (CT scan) or magnetic resonance imaging (MRI) with and without intravenous contrast is a key component to every preoperative routine. Poor quality or inadequate imaging may compromise surgical outcomes and should therefore be repeated prior to discussing management strategies. The surgeon should take special note of tumor characteristics such as size, location especially in relation to the upper, lower, and interpolar regions, hilum and the collecting system (especially the ureter and ureteropelvic junction), and enhancement properties. Additionally, the renal “landmarks” should be identified to aid in intraoperative location of the mass. Other metrics that should be recorded include whether the mass is exophytic (≥50% of mass extending beyond renal contour), mesophytic (20–50% of mass beyond renal contour), or endophytic (<20% beyond renal contour), cystic or solid, enhancement qualities, and abnormalities of shape or contour that may have to be accounted for during TA [25, 26]. Additionally, recent evidence supports the use of the RENAL score as a preoperative metric capable of predicting PN complexity, complication rates, and outcomes [25–38]. While data using the RENAL score for TA is lacking, it may still prove to be useful especially in complicated patients in whom conversion from TA to PN may be necessary. Occasionally, despite the use of high-quality axial imaging, the renal mass is difficult to discern from the surrounding normal renal parenchyma. This can be especially true with endophytic lesions. A preoperative ultrasound of the kidney may help characterize and further delineate the lesion. This may also prove useful since ultrasonography is the primary intraoperative imaging modality utilized in LCA. If the lesion is isoechoic on preoperative ultrasound, it may be difficult to accurately locate at the time of LCA and options should be preoperatively discussed with the patient.
Principles of Ablation
New technologies continue to shape the surgical theater and it is the responsibility of the surgeon to fully understand the method of action, capabilities, and limitations of each new advancement in order to maximize outcomes. This is especially true of TA, which utilizes unique energy delivery systems, different methods of action for tissue destruction, and different targeting and monitoring systems. A proper appreciation for the various treatment modalities portends improved efficacy with a decreased complication rate.
Cryoablation
The current CA systems produce rapid temperature decreases at the probe tip by exploiting the Joule–Thomson principle [39, 40]. At room temperature, all gases with the exception of hydrogen, helium, and neon cool upon expansion. As these gas molecules expand, collision rates between molecules decrease thereby increasing potential energy and decreasing kinetic energy and therefore temperature. Specifically, the modern system utilizes highly pressurized liquid state argon gas that is allowed to expand into the gaseous state near the tip of the probe. The resulting expansion and phase change causes extreme drops in temperature, which induces iceball formation. Iceball dimensions and ablation zones are largely affected by the probe’s design (at what point the gas is allowed to expand and changes in insulation), along with local tissue properties. The iceball does not extend appreciably beyond the tip of the probe but instead extends radially and proximally along the shaft of the probe.
There are several mechanisms that are ultimately responsible for cell death. The rapid cooling initially produces extracellular ice crystal formation followed by intracellular ice crystal formation [39]. The intracellular crystals mechanically disrupt the cell membrane causing dramatic changes in intracellular pH and ionic composition, ultimately leading to protein denaturization. The extreme temperatures bring about local microcirculatory failure, which causes thrombosis, coagulation necrosis, and apoptosis. The dramatic fall in temperature additionally amplifies the extracellular osmotic force resulting in cellular crenation and dehydration. The sum of these effects is uniform cellular death within the ablation zone.
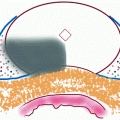
There is not one consistent temperature within the iceball but actually a gradient that extends from −140 to −190°C at the cryoprobe tip to 0°C at the edge of the iceball [41]. This important physical property of the iceball is a main determinant in CA success and failures. While there is extracellular ice crystal formation at the iceball edge, there are no intracellular ice crystals, and it is the intracellular ice that causes cell lysis. Cellular death begins to occur at temperatures below −20°C but is somewhat inconsistent [42]. Uniform and consistent cellular necrosis does not occur until temperatures fall below −40°C. When the iceball temperature gradient is combined with temperature requirements for cell death, three “zones” with different ablation properties are created within the iceball (Fig. 1.3). The central zone extends from the cryoprobe tip to the points within the iceball that are consistently below −40°C. This central zone is characterized by consistent and uniform cellular necrosis. The intermediate zone comprises the iceball area that has reached temperatures between −40 and −20°C and is characterized by both necrotic and viable tissue elements. The outer zone extends from −20°C to the warmer iceball edge and is characterized by mostly viable tissue. It has been determined that temperatures of >−20°C can be measured within 3.1 mm of the iceball edge [43]. Therefore, the standard practice in CA is to extend the iceball to 1 cm beyond the tumor edge to ensure uniform tumor ablation. One of the advantages of LCA is the ability to monitor iceball formation in real time using a laparoscopic ultrasound probe. The expanding iceball creates a readily visualized hyperechoic expanse that delineates the iceball edge (Fig. 1.4). After the freeze cycle is complete, helium or RFA is used to actively thaw the cryoprobe followed by a repeat freeze–thaw cycle.
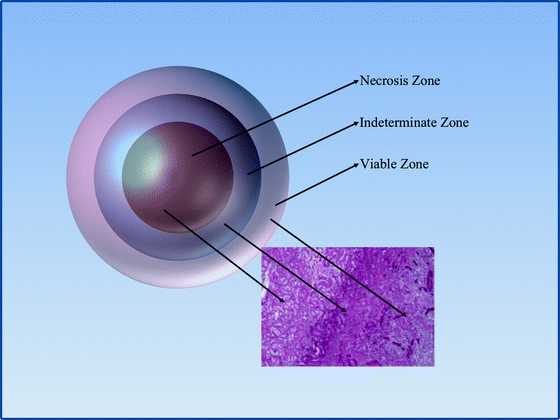
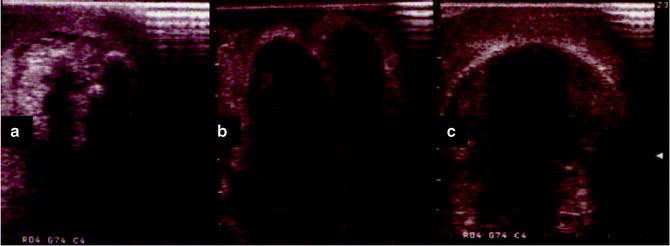

Fig. 1.3
Iceball ablation zones: The central/necrosis zone is characterized by uniform ablation and temperatures <−40°C. Surrounding the central zone is the indeterminate zone which has areas of cell death intermixed with viable cells and temperatures between −40°C and −20°C. The outermost zone is comprised of mostly viable cells with little to no necrosis and temperatures >−20°C

Fig. 1.4
(a) At the start of the freeze cycle, the iceball appears as an expanding hyperechoic region extending radially from the cryoprobes. (b) The hyperechoic regions begin to coalesce as the iceball expands. (c) At the completion of the freeze cycle, the iceball appears as a single hyperechoic mass that extends beyond the margin of the mass
Radiofrequency Ablation
The first report of RFA in the human kidney was in 1997 when Zlotta and colleagues ablated lesions ranging from 2 to 5 cm in three patients [44]. Since then, RFA has gained in popularity as a method to ablate RCN. RFA induces thermal injury by exploiting the resistive properties of the kidney [39]. Probes are introduced into the ablation zone, and high-frequency electrical current is delivered to the target area. The local tissue’s high resistance causes dramatic increases in temperature as the electrical current is transformed into heat. As temperatures climb over 60–100°C, coagulative necrosis results [45, 46]. Irreversible cellular injury does not occur until temperatures reach 50°C; therefore, treatment principles are similar to that of CA with the ablation zone extended to 1 cm beyond the tumor periphery. Unlike CA, however, the ablation zone cannot be precisely visually monitored in real time. To ensure adequacy of treatment, either temperature or impedance probes are placed near the area of interest to determine the extent of effect. At times, this method can be unreliable since eschar formation falsely elevates the readings from impedance probes and temperature probe readings often differ from the true temperature in adjacent parenchyma. RFA has been performed both laparoscopically and percutaneously; however, in the vast majority of available literature, RFA is delivered percutaneously.
Cryoablation Techniques
Maximizing the success of CA involves a combination of appropriate patient selection, understanding, and appropriately applying cryosurgical technology, adhering to the “imaging trifecta,” precise initial probe placement, and accurate iceball management with a willingness to make intraoperative adjustments to any inconsistencies. Patient selection has been discussed elsewhere in this chapter, but in brief, the ideal patient has a mass ≤3 cm in size and has been preoperatively evaluated and counseled appropriately, and the approach has been tailored to the tumor location. The imaging trifecta refers mostly to the laparoscopic approach but certainly pertains to all TA modalities. The first part is the preoperative, high-quality imaging that allows the surgeon to accurately characterize the mass. The second is the liberal use of intraoperative imaging including laparoscopic ultrasound (LCA) or CT scan (PCA) during probe placement. The final aspect is careful iceball monitoring during the freeze–thaw cycles to ensure that the iceball forms as expected with all of the expected margins extending beyond the mass. Correct initial probe placement might be among the most important determinants in success. Once the iceball begins to form, the probe cannot be repositioned, and furthermore, the expanding iceball creates a large acoustic shadow that makes targeting of the deep tissues difficult (Fig. 1.5). Occasionally, local tissue properties and or poor initial probe placement creates an iceball that does not completely ablate the tumor. When this occurs, the surgeon should allow the probes to thaw, reassess, and reposition the probes and perform a repeat cycle to ensure complete tissue destruction.
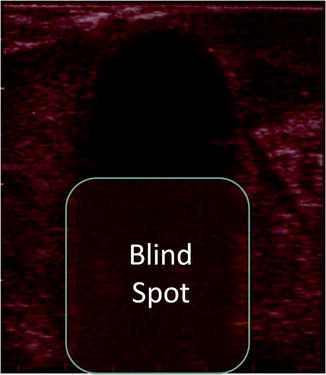

Fig. 1.5
The fully formed iceball obscures the deep margins due to a shadowing effect
Laparoscopic Cryoablation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree