, Sina Tavakoli1 and Sonia L. Betancourt2
(1)
Department of Radiology, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
(2)
Department of Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
32.3.1 Myxoma
32.3.2 Lipoma
32.3.3 Papillary Fibroelastoma
32.3.4 Rhabdomyoma
32.3.5 Fibroma
32.3.6 Paraganglioma
32.4.1 Sarcomas
32.4.2 Angiosarcoma
32.4.3 Rhabdomyosarcoma
32.4.5 Lymphoma
32.7 Summary
Abstract
Primary cardiac neoplasms are rare and identified in 0.05–0.15 % of cases in large autopsy [1, 2] and echocardiography series [3]. Primary cardiac tumors may be classified as benign or malignant. The most common benign cardiac tumors include myxoma, rhabdomyoma, fibroma, and lipoma, whereas the most common malignant primary tumors are sarcomas (angiosarcoma, fibrosarcoma, undifferentiated sarcoma, rhabdomyosarcoma) and lymphoma [4, 5]. The majority, about three-fourth, of these tumors are benign [1, 2]. Secondary involvement of the heart or pericardium through local invasion or metastatic spread of malignant tumors is at least 20 times more common, identified in about 1.2 % of autopsy cases [1, 2] and 8 % of cancer victims [6]. Thoracic tumors (e.g., lung, breast, and esophagus) commonly invade the pericardium and heart. Other tumors that tend to metastasize to the heart include melanoma, soft tissue sarcomas, and gastric, pancreatic, thyroid, renal, and hepatocellular carcinomas [2, 6]. Cardiac involvement in lymphoma and leukemia is also common [2, 6]. When analyzed by the frequency according to the histologic type of the primary tumor, melanoma is the malignancy that more often metastasizes to the heart (46 % in autopsy series), followed by germ cell tumors (38 %), leukemia, and lymphoma [7].
Abbreviations
ADC
Apparent diffusion coefficient
CT
Computed tomography
DWI
Diffusion weighted imaging
ECG
Electrocardiography
18F
18Fluorine
18F-FDG
Fluorodeoxyglucose
123I-MIBG
123I-metaiodobenzylguanidine
MRI
Magnetic resonance imaging
PET
Positron emission tomography
RSE
Maximum relative signal enhancement
SPIR
Spectral presaturation by inversion recovery
SUVmax
Standard uptake value
TSE
Turbo spin echo
Primary cardiac neoplasms are rare and identified in 0.05–0.15 % of cases in large autopsy [1, 2] and echocardiography series [3]. Primary cardiac tumors may be classified as benign or malignant. The most common benign cardiac tumors include myxoma, rhabdomyoma, fibroma, and lipoma, whereas the most common malignant primary tumors are sarcomas (angiosarcoma, fibrosarcoma, undifferentiated sarcoma, rhabdomyosarcoma) and lymphoma [4, 5]. The majority, about three-fourth, of these primary tumors are benign [1, 2]. Secondary involvement of the heart or pericardium through local invasion or metastatic spread of malignant tumors is at least 20 times more common, identified in about 1.2 % of autopsy cases [1, 2] and 8 % of cancer victims [6]. Thoracic tumors (e.g., lung, breast, and esophagus) commonly invade the pericardium and heart. Other tumors that tend to metastasize to the heart include melanoma, soft tissue sarcomas, and gastric, pancreatic, thyroid, renal, and hepatocellular carcinomas [2, 6]. Cardiac involvement in lymphoma and leukemia is also common [2, 6]. When analyzed by the frequency according to the histologic type of the primary tumor, melanoma is the malignancy that more often metastasizes to the heart (46 % in autopsy series), followed by germ cell tumors (38 %), leukemia, and lymphoma [7].
Cardiac neoplasms may be incidentally diagnosed in imaging work-up performed for other purposes or result in symptoms by either interfering with normal cardiac physiology (e.g., valvular dysfunction, impaired diastolic filling of the chambers, contractile dysfunction, arrhythmias, and pericardial effusion/tamponade) or distal embolization [8, 9]. In general, tumor location, rather than its histopathology, is more important in determining the clinical presentation of cardiac neoplasms. Extra-cardiac extension of the tumors may also produce symptoms related to compression of lungs and airways (e.g., dyspnea) or esophagus (dysphagia). Additionally, some cardiac tumors, e.g., myxoma, may produce constitutional symptoms such as fever, weight loss, and fatigue [8, 9].
32.1 Cardiac Imaging: Anatomy and Physiology
Plain radiography is neither sensitive nor specific for detection of cardiac neoplasms, but large tumors may produce an abnormal cardiac silhouette on chest radiographs. Cardiomegaly, pulmonary edema, pericardial effusion, extra-cardiac tumor growth, and occasionally tumor calcifications may be identified in plain radiographs.
Transthoracic echocardiography is an inexpensive, noninvasive, and widely available modality for evaluation of cardiac neoplasms. It allows real-time assessment of cardiac anatomy and physiology with a high spatial and temporal resolution. Therefore, tumor mobility and its physiological consequences, e.g., valvular or contractile dysfunction, can be readily evaluated. However, the operator dependency of echocardiography and poor acoustic window frequently challenge thorough evaluation of the heart, especially in obese people or patients with obstructive pulmonary disease. Transesophageal echocardiography is an alternative and complementary modality which allows high-resolution imaging of cardiac structures, by using higher-frequency transducers and providing a better acoustic window. Low soft tissue contrast and limited visualization of the extra-cardiac structures, including the great vessels and other mediastinal structures, remains a major a limitation of transthoracic and transesophageal echocardiography.
Development of electrocardiography (ECG)-gated multi-detector scanning techniques and fast imaging sequences has evolved the role of CT and MRI in evaluation of cardiac diseases, including cardiac tumors, over the past two decades. Both modalities are excellent in visualization of mediastinal structures and demonstration of extra-cardiac extent of tumor and have a markedly better soft tissue contrast compared to echocardiography, and thus improve structural characterization of the tumor. The superior soft tissue contrast, lack of ionizing radiation, and multiplanar capability of MRI have made it the most comprehensive technique for cardiac tumor imaging. Additionally, the extent of myocardial infiltration can be more accurately determined by MRI, in particular following contrast administration. Cardiac CT angiography is invaluable in the assessment of coronary arteries which is critical in surgical planning of patients with epicardial tumors.
Echocardiography, CT, and MRI provide morphological details of tumors, their extra-cardiac extension, and their physiological consequences. However, most often a specific histological diagnosis cannot be reached by these modalities. Unlike malignant cardiac neoplasms, benign tumors can often be observed or successfully resected. Unfortunately, the critical location of cardiac tumors and high risk of serious complications may overweigh the diagnostic benefits of invasive approaches such as catheter-guided myocardial biopsy. Therefore, differentiation of benign and malignant cardiac tumors is an important clinical issue in patient management, in particular in determining the resectability of the tumor and surgical approaches. Several morphologic features, including an infiltrative growth pattern, tumor heterogeneity, and the presence of pericardial effusion, have been suggested to differentiate malignant and benign cardiac neoplasms [10]. However, the accuracy of these criteria either alone or in combination remains suboptimal [10].
32.2 Cardiac Imaging: Function and Metabolism
Imaging tumor metabolism is a powerful tool in diagnosis and monitoring treatment response in oncology. 18Fluorine(18F)fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) is the most commonly used metabolic imaging technique in imaging of patients with malignant neoplasms. 18F-FDG is a glucose analogue in which the hydroxyl group at the 2 position of the glucose is substituted with the positron-emitting radionuclide 18F. The uptake of 18F-FDG by cells through glucose transporters followed by its hexokinase-mediated phosphorylation results in the formation of cell membrane-impermeable product 6-deoxyglucose which is unable to proceed through the remainder of the glycolytic pathway and therefore will be trapped in the cells. The preferential utilization of glucose through aerobic glycolysis in malignant tumors, known as Warburg effect, is associated with enhanced 18F-FDG uptake which is the basis of widespread application of PET in oncology. However, high physiological uptake of glucose by normal myocardium significantly limits the utility of 18F-FDG PET imaging in cardiac neoplasms, where its application has been mostly limited to a few case reports and small case series [10, 11].
Physiological glucose uptake by myocardium necessitates a special patient preparation protocol for evaluation of cardiac neoplasms [11]. In patients who undergo 18F-FDG PET imaging for evaluation of myocardial viability, a glucose loading protocol is typically employed to enhance myocardial 18F-FDG uptake [12]. This is in contrast to PET imaging protocol performed in oncologic patients in which 18F-FDG is administered after at least 6 h of fasting to reduce the glucose uptake by nonmalignant cells, including normal myocardial tissue [12]. After a prolonged fasting (>12 h), myocardial metabolism shifts to fatty acid utilization and therefore 18F-FDG uptake will be suppressed [12, 13]. However, the duration of fasting is usually shorter in most clinical studies performed in oncologic patients. This causes a nonuniform suppression of myocardial 18F-FDG uptake with focal and regional areas of increased uptake which may mimic cardiac neoplasms [11–13]. Typically, the heart base is the last area to switch from glucose metabolism to fatty acid utilization [13]. The nonuniform myocardial 18F-FDG uptake and its normal physiological variation which may exceed the difference between benign and malignant tumors should be carefully analyzed in the evaluation of cardiac diseases to avoid erroneous diagnosis of cardiac tumors [11]. Additionally, increased 18F-FDG uptake may be seen in a number of nonneoplastic cardiac conditions including inflammatory and infectious diseases (e.g., myocarditis and sarcoidosis), ischemic heart disease, congestive heart failure, and atrial fibrillation [11–13]. Considering the significantly lower incidence of cardiac tumors relative to these conditions, abnormal myocardial 18F-FDG uptake is likely attributable to these nonneoplastic conditions [11]. However, the pattern of increased 18F-FDG uptake in these diseases is usually different from patients with cardiac neoplasms, and multimodality imaging helps in accurate determination of nonneoplastic nature of abnormal 18F-FDG uptake [11–13].
A recent case series with 24 patients diagnosed with primary or secondary cardiac neoplasms has shown 2.5–3-folds higher 18F-FDG uptake in metastatic and primary malignant cardiac tumors compared to primary benign cardiac neoplasms, suggesting that 18F-FDG PET imaging may improve the noninvasive preoperative distinction of benign and malignant neoplasms in selected patients with known cardiac tumors [10]. This study demonstrated a wide range of SUVmax among cardiac neoplasms ranging from negligible uptake (SUVmax = 1.6 in a patient with myxoma) to a markedly increased uptake (SUVmax = 16.7 in a patient with cardiac lymphoma) [10]. Unlike metastatic and primary malignant cardiac tumors, benign cardiac neoplasms had no positive visual contrast compared to the adjacent normal myocardium [10]. The authors have concluded that a cutoff SUVmax value of 3.5 may be used for distinction between benign and malignant cardiac tumors with sensitivity and specificity of 100 and 86 %, respectively, in this selected population of patients with known cardiac neoplasms [10]. The major limitations of this study, as identified by the authors, are the retrospective nature of the study and semiquantitative nature of the SUVmax which is influenced by blood glucose and insulin levels as well as technical factors including the imaging equipment. However, the results of this study are very promising and suggest that 18F-FDG PET imaging can complement the diagnostic work-up of patients with cardiac neoplasms.
In the following sections, we will review the most common cardiac neoplasms and their imaging findings including available data on functional imaging.
32.3 Benign Primary Cardiac Neoplasms
32.3.1 Myxoma
Myxoma is the most common primary cardiac neoplasm, accounting for 15 and 50 % of cases in pediatric and adult patients, respectively [8, 9]. The majority of cases are sporadic and present in the fourth to sixth decades of life [9] with a female to male ratio of about 3 to 2 [14]. Approximately 7 % of cases are associated with Carney complex, a rare autosomal dominant syndrome [9]. These patients are younger and present with pigmented skin lesions, endocrinopathies, and a variety of tumors including breast fibroadenomas, psammomatous melanotic schwannomas, and osteochondromyxoma [9]. Cardiac myxoma is an incidental imaging finding in about 10–20 % of patients [15]. The classic triad is obstructive symptoms from mitral or tricuspid stenosis (67 %); constitutional symptoms, e.g., fever, malaise, and weight loss (34 %); and embolic complications (29 %) [16, 17]. Embolic events are more common in left-sided tumors and may result in stroke, renal, and splenic infarcts or limb ischemia [18].
Myxomas are benign gelatinous tumors composed of endothelial and smooth muscle cells and intermediate cellular forms derived from multipotential mesenchymal cells which are scattered in a mucopolysaccharide stroma [19]. Tumors may become quite large and are typically located in the left (75 %) or right (20 %) atria [9, 19]. They most often appear as pedunculated intracavitary masses attached to the interatrial septum, usually at fossa ovalis [8, 16]. Ventricular origin or bi-atrial growth through the fossa ovalis is rare [16]. Myxomas associated with Carney complex are more likely to be multiple or atypical in location and recur following resection [9]. About one-third of myxomas have a pliable surface with a propensity for embolization [20].
The location, narrow stalk, and mobility are the key imaging findings. The typical atrial location of the myxoma and its origination from the interatrial septum can usually be depicted in all imaging modalities. However, the narrow stalk and mobility of the tumor are best appreciated on echocardiography. On echocardiography, myxomas may be homogeneous or contain heterogeneous foci representing hemorrhage, necrosis, and calcification [9]. On CT, myxomas are usually heterogeneous (67 %) and hypoattenuating (81 %) to isoattenuating (19 %) compared to the myocardium (Fig. 32.1) [9, 15, 16]. Calcification is uncommon and detected in only about 14 % of cases (Fig. 32.2) [15]. Tumors are usually hypo- to isointense to myocardium on T1-weighted images and bright on T2-weighted images, reflecting their gelatinous composition [8, 16, 18]. Tumor heterogeneity is best appreciated by MRI and becomes more conspicuous after contrast administration and is present in about 90 % of patients [8, 15]. PET imaging demonstrates low without significant contrast compared to the normal myocardium which aids in the diagnosis of atypical of myxomas [10, 21].
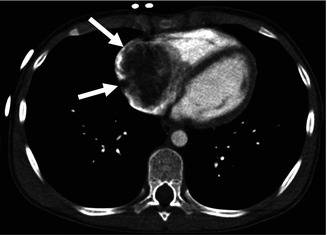
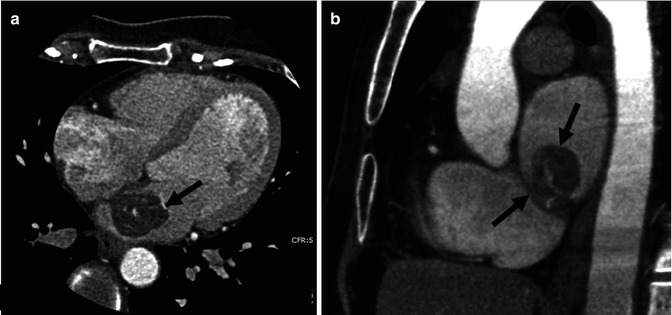

Fig. 32.1
Right atrial myxoma in a 14-year-old male. Contrast-enhanced CT shows a large low-density mass occupying the right atrium (arrows)

Fig. 32.2
Left atrial myxoma in a 55-year-old female. Contrast-enhanced cardiac-gated CT angiogram. (a) Axial image. (b) Sagittal image. The attachment of the mass (arrows) to the interatrial septum is appreciated on both axial and sagittal planes. Small dense focal areas of calcification within the mass are noted
32.3.2 Lipoma
Lipoma is probably the second most common primary cardiac tumors in adults [18], though its prevalence may have been overestimated as in some studies lipoma and lipomatous hypertrophy of the interatrial septum have not been separately reported [9]. Cardiac lipomas are usually sporadic and occur over a wide range of age. There is an increased incidence of cardiac lipoma in patients with tuberous sclerosis in whom multiplicity is common [8, 9].
Patients are usually asymptomatic, but large tumors may interfere with filling of cardiac chambers and valvular function or compress lungs and airways [8, 9]. Arrhythmia may occur due to infiltration of the conduction system [9]. Surgical resection may be required due to symptoms and progressive growth.
Cardiac lipomas are benign encapsulated neoplasms [9] which may originate from any of the three cardiac layers, i.e., epicardium, myocardium, or endocardium [8]. Intracavitary lipomas commonly arise from the interatrial septum, but unlike myxomas, are broad based [9]. Less commonly lipomas may arise from the valves. The fatty composition of the tumor can be readily diagnosed by CT and MRI (Fig. 32.3) [8, 9, 18]. There is little or no contrast enhancement [8, 9, 18] and low 18F-FDG uptake [21].
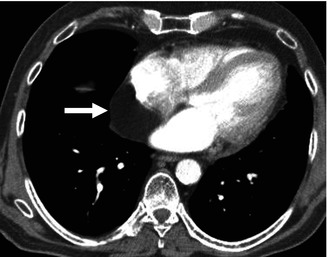

Fig. 32.3
Cardiac lipoma. Contrast-enhanced CT shows a fat density mass within the posterior wall of the right atrium (arrow)
Lipomas that originate from the interatrial septum may resemble lipomatous hypertrophy of the septum, defined as nonneoplastic septal deposition of fat exceeding 2 cm in thickness [9]. Unlike cardiac lipomas, lipomatous hypertrophy of the interatrial septum typically spares the fossa ovalis resulting in a dumbbell-shaped lesion (Fig. 32.4) [22] and demonstrates marked 18F-FDG uptake [13, 23] likely related to the brown fat content of the latter condition [23]. Interestingly, 18F-FDG uptake may be intermittent in patients with lipomatous hypertrophy of the interatrial septum [24]. Occasionally, modest amount of fat in the interatrial septum can also exhibit increased metabolic uptake (Fig. 32.5). Hypermetabolic brown fat may also localize in the pericardium and other mediastinal compartments and be misinterpreted as a primary tumor or metastatic disease in cancer patients [25].
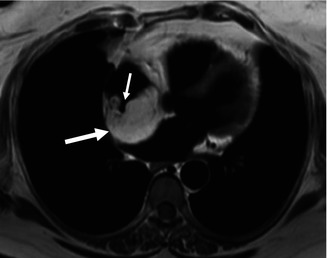
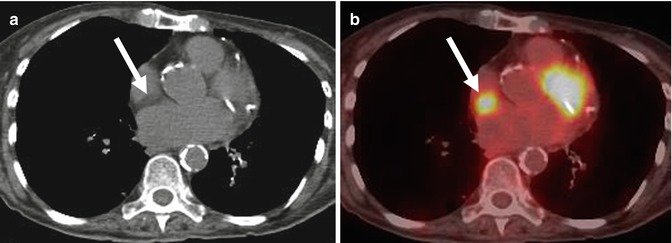

Fig. 32.4
Lipomatous hypertrophy of the interatrial septum in a 60-year-old obese female patient. Cardiac MR, black blood axial image reveals a large mass within the interatrial septum (large arrow) isointense with the subcutaneous fat. The mass spares the fossa ovalis which manifests as a channel-like region which opens into the right atrium (small arrow)

Fig. 32.5
Hypermetabolic fat in the interatrial septum. (a) Noncontrast CT shows moderate amount of low-density fat in the interatrial septum (arrow). (b) 18F-FDG PET/CT fusion image shows hypermetabolic activity at the same level (arrow) consistent with brown fat
32.3.3 Papillary Fibroelastoma
Papillary fibroelastoma constitutes approximately 10 % of primary cardiac neoplasms in adults [9, 16, 26] and comprises over 70 % of primary tumors arising from cardiac valves [27]. The mean age of presentation is 60 years without a significant gender predilection [28, 29]. One-third of patients are asymptomatic [28, 29]. Symptoms are more common in left-sided tumors and are usually caused by embolization of tumor fragments or overlying thrombi resulting in stroke, transient ischemic attack, or myocardial infarction [28, 29].
Papillary fibroelastoma arises from the endocardial layer and is composed of fronds of myxoid connective tissue covered by endothelium [9, 19]. Its gross morphology has been resembled to sea anemone [9]. Tumors are usually single and smaller than 2 cm [9]. More than 80 % of tumors emanate from valvular surfaces, in order of frequency: aortic (29 %), mitral (25 %), tricuspid (17 %), and pulmonic (13 %) [9, 30]. Less frequently (16 %) tumors may arise from non-valvular endocardial surface [9]. Multiple autopsy series have demonstrated almost equal distribution of fibroelastomas in right- and left-sided cardiac chambers, raising the question that the apparent higher prevalence of tumors arising from the left-sided valves in clinical series may be related to their tendency to be symptomatic [31].
Various hypotheses have been suggested to explain pathogenesis of papillary fibroelastoma, including true neoplastic or hamartomatous growth, abnormal endocardial reaction to chronic hemodynamic stress, and organization of thrombi [31]. Fibroelastomas appear to be iatrogenic and related to radiation or cardiac surgery in about 20 % of patients. A non-valvular distribution of tumor is more frequent in these cases [31].
Due to their small size and attachment to cardiac valves causing their mobility, fibroelastomas are best evaluated on echocardiography and may be difficult to be identified by other imaging modalities, including CT, MRI, and PET. Pedunculated morphology and filiform projections distinguish them from amorphous thrombi attached to cardiac valves [9]. More recently, with the improved spatiotemporal resolution of cardiac-gated CT, small papillary fibroelastomas can be readily detected with this modality (Fig. 32.6). Calcification is rare [9]. Tumors are usually hypointense on T2-weighted images [8, 32] and may cause turbulent flow on cine MRI sequences [18]. In a recent report, low 18F-FDG uptake was reported in a patient with cardiac fibroelastoma [10].
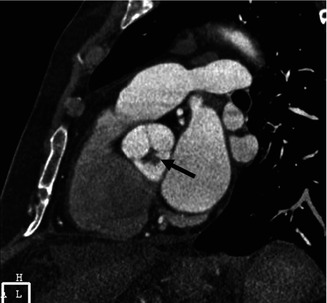

Fig. 32.6
Papillary fibroelastoma of the aortic valve. Gated cardiac CT, double-oblique image at the sinus of Valsalva shows a lobulated small mass attached to the aortic valve (arrow)
32.3.4 Rhabdomyoma
Rhabdomyomas are the most common intracardiac tumor in children comprising about 90 % of primary benign cardiac tumors in this age group and are usually identified in late pregnancy or infancy [33]. They are associated with tuberous sclerosis in about 50 % of patients [33]. Asymptomatic patients can be observed, as most lesions tend to regress spontaneously [33]. Resection may be required to treat symptoms resulting from obstruction of the inflow or outflow tract and arrhythmias [33].
Rhabdomyomas are firm circumscribed hamartomatous nodules and are histologically characterized by a mixed population of abnormal myocytes containing large glycogen vacuoles [19, 31, 33]. Multiplicity is common in patients with tuberous sclerosis (>90 %) [8, 31, 33]. Sporadic rhabdomyomas are usually larger than those associated with tuberous sclerosis [33]. They may occur anywhere in the heart and pericardium, but are more common in the ventricles [31, 33]. Hemorrhage and calcification are uncommon [33].
Cardiac rhabdomyomas typically appear as small lobulated and homogeneously echogenic masses arising from the interventricular septum [34]. Lesions are hypo- or hyperattenuating in CT, isointense to slightly hyperintense on T1-weighted and hyperintense on T2-weighted sequences (Fig. 32.7) [8]. Cardiac rhabdomyomatosis is a rare condition characterized by myocardial infiltration by numerous microscopic lesions appearing as diffuse myocardial thickening which may cause cardiomyopathy [8, 31]. To our knowledge 18F-FDG uptake has not yet been reported in cardiac rhabdomyoma.
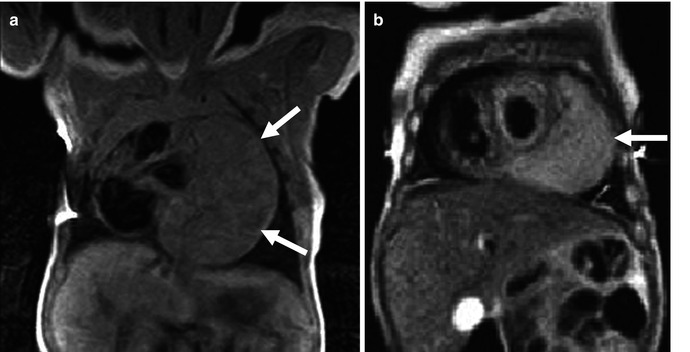

Fig. 32.7
Cardiac rhabdomyoma in a newborn male with tuberous sclerosis. Cardiac MR, black blood images demonstrates a large mass on the anterolateral aspect of the left side of the heart (arrows) isointense on T1 (a) and slightly hyperintense on T2-weighted sequences (b)
32.3.5 Fibroma
Fibroma is the second most common primary cardiac tumor in children after rhabdomyoma and usually presents in perinatal period or in the first years of life [8], though it may be diagnosed in older patients in about 15 % of cases [15, 35]. About one-third of cardiac fibromas are asymptomatic [9]. Unlike rhabdomyomas, spontaneous regression does not occur [33], and resection is generally required as tumors are usually large (average size of 5 cm) and may cause heart failure, arrhythmias, or sudden death [8, 9, 18]. The incidence of cardiac fibroma is higher in patients with Gorlin syndrome, an autosomal dominant condition characterized by developmental abnormalities and predisposition to multiple neoplasms including basal cell carcinoma and odontogenic keratocysts [9, 36].
Fibromas are usually solitary fibrous lesions within the left ventricular free wall or septum [15, 33]. Atria and right ventricle are uncommon sites of involvement [33]. Unlike rhabdomyomas, calcification is common in fibromas, and cystic degeneration or necrosis may be seen especially in large tumors [8, 33, 37]. Histologically, fibromas are not encapsulated and are composed of fibroblasts scattered in a collagenous matrix [9, 31].
On echocardiography, cardiac fibromas present as noncontractile homogenous ventricular masses which may resemble focal hypertrophic cardiomyopathy [9]. On cross-sectional imaging, these tumors usually appear as homogeneous soft tissue masses which may be infiltrative or circumscribed (Fig. 32.8). Due to their fibrous nature, they are usually isointense to hypointense to the myocardium on T1- and T2-weighted images and demonstrate delayed contrast enhancement [8, 9, 38]. Calcifications are common and best depicted by CT (Fig. 32.9). A recent report demonstrated low 18F-FDG uptake in a patient with cardiac fibroma with SUVmax of 3.3 [10].
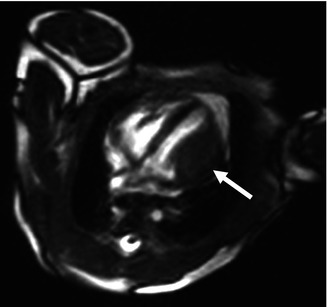
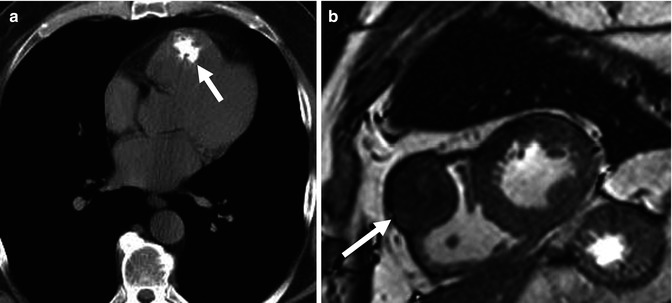

Fig. 32.8
Cardiac fibroma in a newborn. White blood axial MR image shows a hypointense fusiform mass on the lateral wall of the left ventricle (arrow)

Fig. 32.9
Calcified right ventricular fibroma in a 49-year-old male. (a) Noncontrast CT shows an irregular calcification in the anterior aspect of the right ventricle (arrow). (b) Short-axis white blood cardiac MR confirms the presence of a hypointense mass within the anterior right ventricular wall (arrow)
32.3.6 Paraganglioma
Cardiac paragangliomas are extremely rare and are usually diagnosed in young adults [9, 39]. Almost all cases are sporadic [9] and may occur synchronous with extra-cardiac paragangliomas [9




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree