Fig. 53.1
(a) Whole-body FDG-PET maximum intensity projection (MIP), (b) axial PET, (c) PET/CT fused, and (d) corresponding contrast-enhanced CT images in a 51-year-old patient with diffuse large B-cell lymphoma (DLBCL). FDG-PET helped to highlight a supraclavicular involvement in a centimetric node (arrows), which can be easily overlooked on the diagnostic contrast-enhanced CT. Note extensive nodal and extranodal involvement, all below the diaphragm
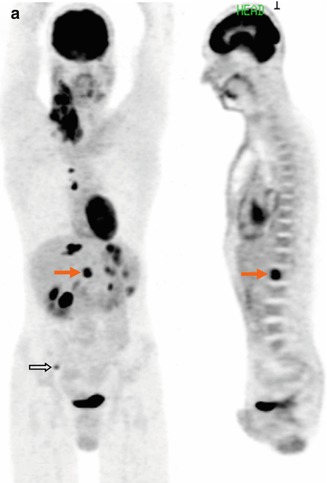
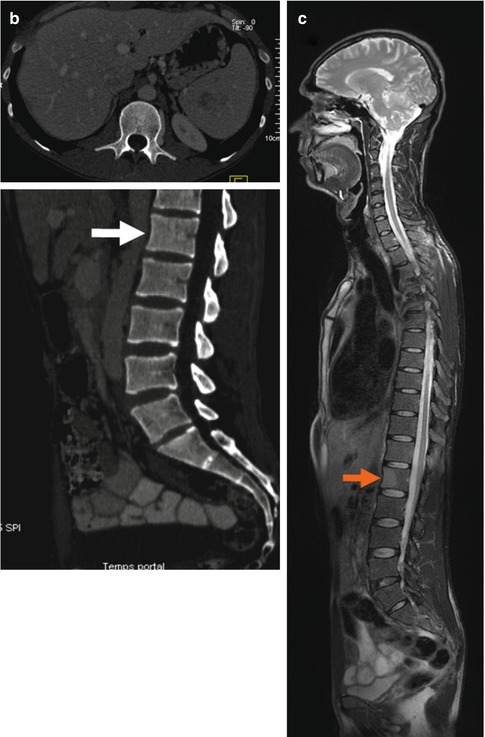
Fig. 53.2
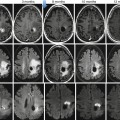
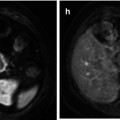
(a) Whole-body FDG-PET MIP and sagittal multi-planar reconstruction (MPR) images clearly detected extranodal marrow involvement within the L1 vertebral body (arrows) in a 35-year-old patient with Hodgkin lymphoma (HL), as well as another tiny lesion in right iliac crest (open arrow). The L1 lesion was missed on diagnostic CT (b) and was confirmed on unenhanced T2-weighted MR image (c)
For response assessment of lymphoma patients, contrast-enhanced CT of the thorax, abdomen, and pelvis has been the principal modality with the International Working Group (IWG) criteria for response assessment in therapeutic trials [12, 16]. However, disease is primarily assessed on the basis of size criteria. A major problem in the interpretation of response to treatment has been that persistence of residual masses on diagnostic CT after chemotherapy does not necessarily indicate residual disease (Fig. 53.3). This problem is more pronounced in patients who have huge masses (bulky disease) at initial presentation [12]. Therefore, development of functional imaging approaches capable of differentiating fibrosis from viable tumor within residual masses is of utmost importance in the lymphoma patient management. Dugdale et al. showed that perfusion measurements from dynamic contrast-enhanced (DCE) CT were higher in patients with active residual masses and aggressive NHL [17]. In addition, perfusion fell when disease became inactive; therefore, CT perfusion measurements have potential for assessing lymphoma grade, activity, and treatment response [17] (Fig. 53.4). However, CT perfusion study has the radiation issue. MRI does not require radiation and provides excellent soft tissue contrast, which may help overcoming this issue (see Sect.53.5).
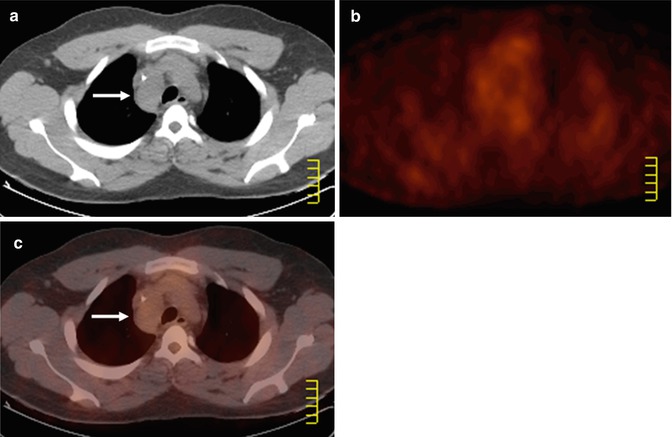
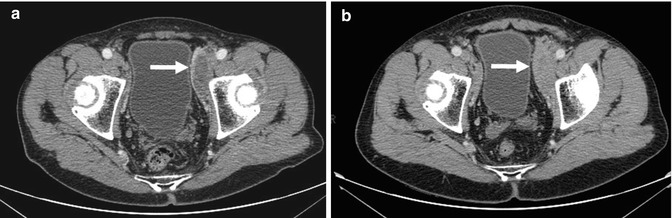

Fig. 53.3
Axial (a) co-registered CT, (b) FDG-PET, and (c) PET/CT fused images in a 20-year-old patient with DLBCL at treatment completion. The residual right paratracheal mass (arrows, a, c, classified as partial response based on CT criteria) shows no increased glucose metabolism, and the patient remained in complete remission status

Fig. 53.4
A 74-year-old patient with mantle cell lymphoma presented with persistent residual masses at the end of therapy (a, arrow). Follow-up contrast-enhanced CT images later show increased enhancement of the left external iliac nodal mass from (a) to (b, arrow) when the patient had clinical disease relapse
53.3 Scintigraphy
Until the introduction of PET and SPECT (single photon emission computed tomography), several metabolic imaging strategies using scintigraphy were applied in patients with lymphomas. Before the PET era, gallium-67 (67Ga) scintigraphy was shown to be useful in assessing response in lymphoma, improving on the specificity of CT, especially in the post-therapy setting for the evaluation of residual masses that are indeterminate for lymphoma versus necrosis [18–20]. 67Ga scintigraphy relies on the accumulation of the isotope into viable lymphoma cells by binding to transferrin receptors. However, 67Ga scanning was not widely adopted because its spatial resolution, specificity, and sensitivity were low for indolent lymphomas and abdominal disease due to physiological bowel uptake [21–23]. For certain types of lymphomas such as follicular or mantle cell lymphomas, sensitivity of gallium scanning can be as low as 30 % [24]. The time involved in performing the scans (patients imaged 2–3 days after 67Ga injection) further limited its use [23].
53.4 PET and PET/CT
PET and/or PET/CT using fluorine-18 fluorodeoxyglucose (FDG) has been demonstrated not only to have significantly higher tumor-to-background ratios than gallium scanning [25] but also to be more sensitive and specific than 67Ga scintigraphy in depicting lymphoma lesions [23]. As mentioned earlier, CT has been the gold standard for the staging of lymphomas but offers only structural information. In lymphomas, PET scans have been evaluated in pretreatment staging, restaging, monitoring during therapy, and the assessment of transformation. The integrated PET/CT system provides both functional and anatomical information in a single examination, minimizing the problem of co-registration related to differences in patient positioning, bed profiles, and internal organ movement, and has become the mainstream in recent years.
53.4.1 Staging
Several studies have shown that combined PET and low-dose, noncontrast CT scanning has better diagnostic performance than diagnostic contrast-enhanced CT scans. According to Schaefer et al., sensitivity of PET/CT and contrast-enhanced CT for evaluation of lymph node involvement in 60 patients with Hodgkin or high-grade non-Hodgkin lymphoma was 94% and 88 %, and specificity was 100 and 86 % [15]. For evaluation of organ involvement, sensitivity of PET/CT and contrast-enhanced CT was 88 and 50 %, and specificity was 100 and 90 %, respectively. In another series of 87 patients, PET/CT detected additional lesions over diagnostic CT in 30 patients, of which 11 demonstrated increased Ann Arbor clinical stage, therefore resulting in therapy change in 2 patients [26]. If CT only had been used, no change of stage or of therapy would have been proposed. The principal advantages of functional PET information over diagnostic CT were the disease detection within normal-sized lymph nodes (Fig. 53.1), unexpected locations related to excellent lesion-to-normal tissue contrast (Fig. 53.5) and anatomical coverage (Fig. 53.6), and most important of all the detection of extranodal involvement (Fig. 53.7). However, the sensitivity of FDG-PET may be compromised in case of low-grade lymphoma subtypes, bone marrow infiltrates by small lymphoid cells (Fig. 53.8) [27], and renal involvement (Fig. 53.9).
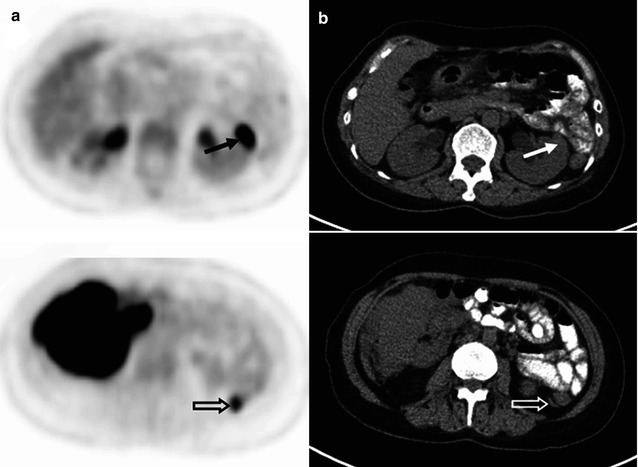
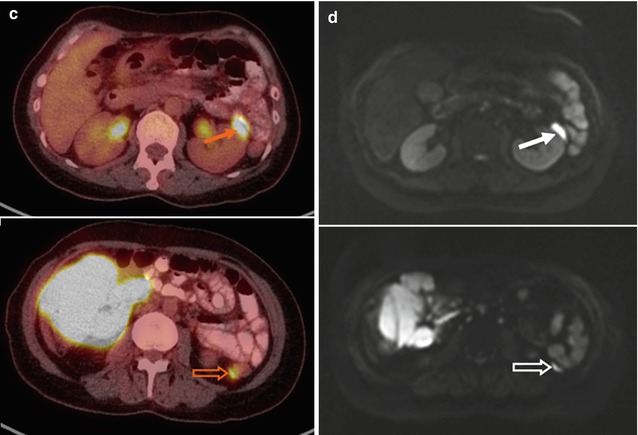
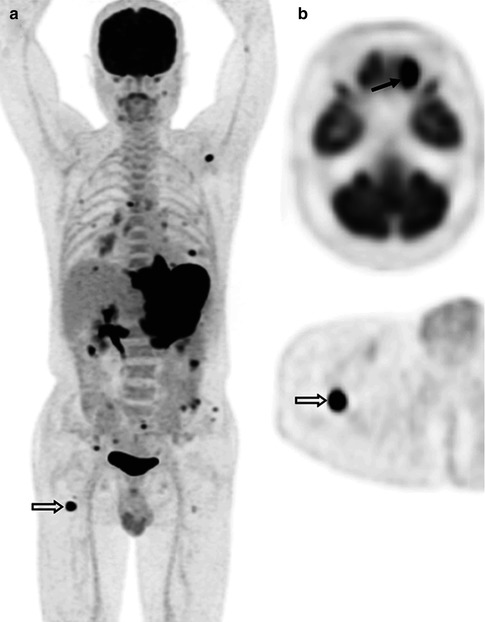
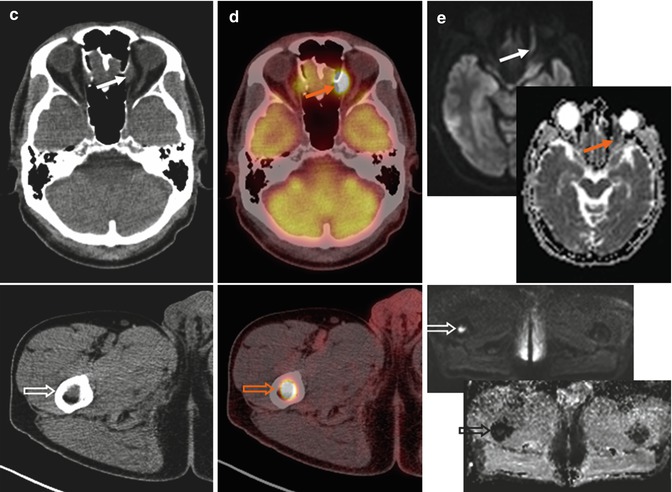
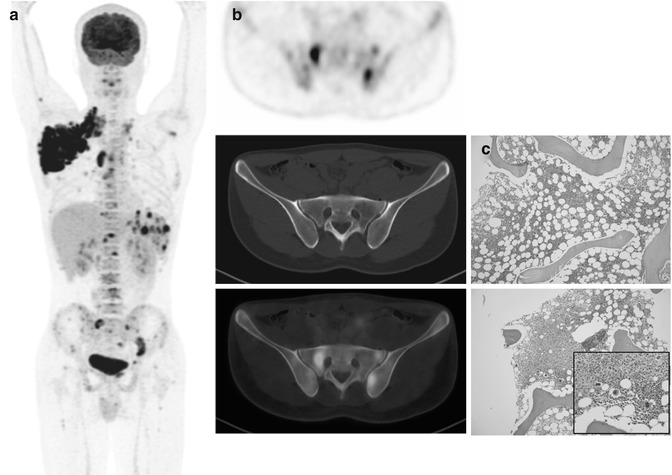
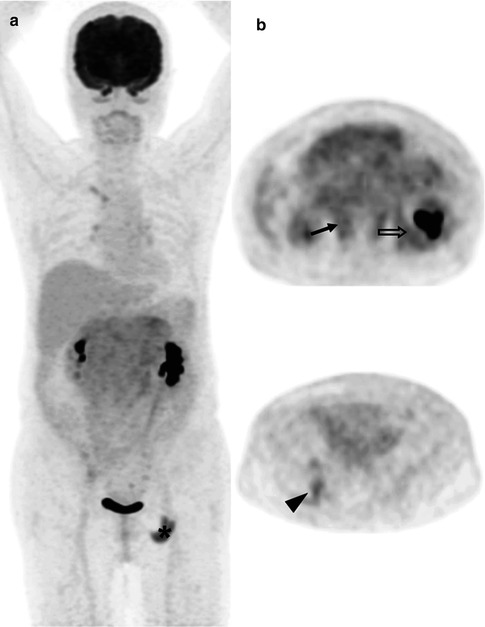
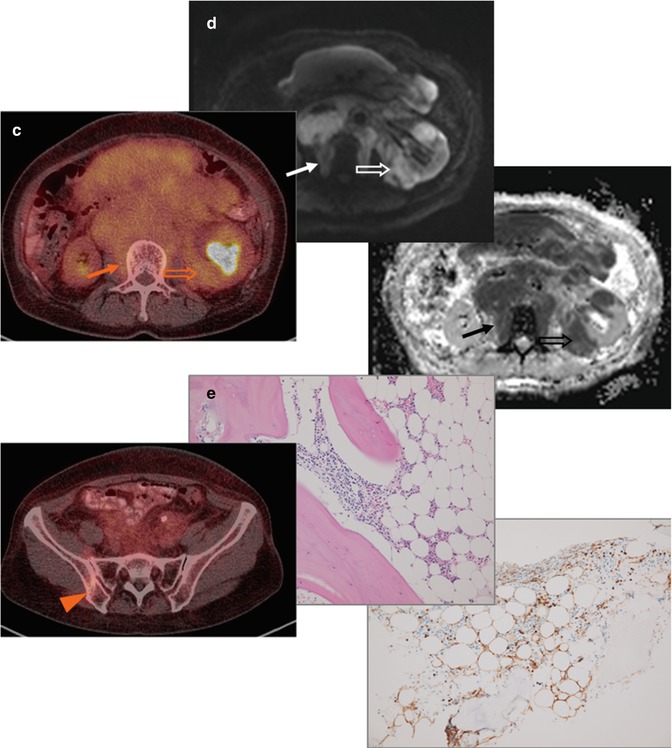
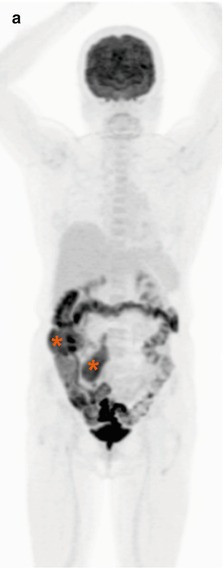
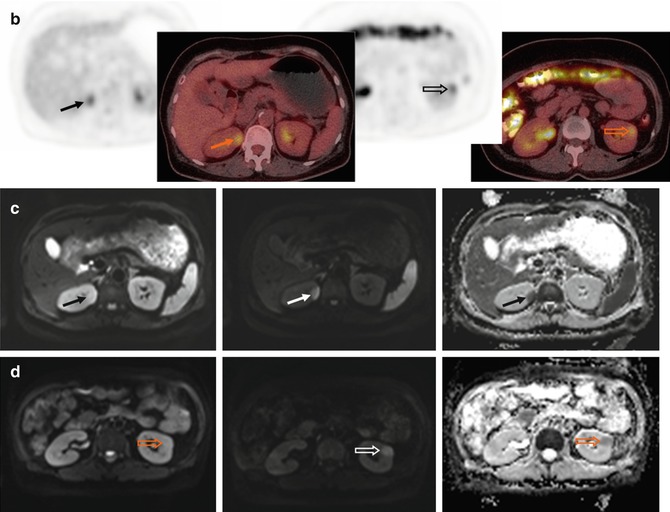


Fig. 53.5
(a) FDG-PET, (b) CT, and (c) PET/CT fused images demonstrate two thin soft tissue lesions with markedly increased uptake at left anterior pararenal space (arrows) and posterior to the descending colon (open arrows) in a 46-year-old patient with DLBCL. These two lesions are both hyperintense on (d, arrow, open arrow) diffusion-weighted (DW) images with a b-value of 800 s/mm2, i.e., with diffusion restriction. Note also bulky disease over right-sided abdomen


Fig. 53.6
(a) Whole-body FDG-PET MIP, (b) axial PET, (c) CT, (d) PET/CT fused, (e) DW images with a b-value of 800 s/mm2 and their corresponding apparent diffusion coefficient (ADC) parametric maps in a 64-year-old patient with DLBCL. FDG-PET/CT depicts rare location of lymphoma involvement within left orbit (arrows), where air in the adjacent paranasal sinuses and a location at the edge of its anatomical coverage may cause distortion on the DW images making diagnosis difficult. Bone marrow lesions are better visualized on high-b DW images since normal marrow already has restricted diffusion on the ADC map (open arrows). Note bulky disease involving the gastric area and multifocal bowel and bone/bone marrow involvement readily identified on FDG-PET due to excellent lesion-to-background contrast

Fig. 53.7
(a) Whole-body FDG-PET MIP, (b) axial PET/CT images at the posterior iliac crest level (bone marrow biopsy site), and (c) microscopic photographs (hematoxylin and eosin, original magnification 10× and inset, 20×) in a 17-year-old patient with HL. FDG-PET/CT shows nodal, splenic, and multifocal bone/bone marrow involvement. The initial bone marrow biopsy performed on the right iliac crest (c: upper) was negative for lymphoma involvement. Repeat left iliac crest biopsy confirmed the involvement of Hodgkin/Reed–Sternberg-like cells (c: lower with inset)


Fig. 53.8
(a) Whole-body FDG-PET MIP, (b) axial PET, and (c) PET/CT images at renal hilar (upper) and posterior iliac crest (lower) levels, (d) DW images with a b-value of 800 s/mm2 and its corresponding ADC map in a 61-year-old patient with grade 1–2 follicular lymphoma. DW image and ADC map better delineate tumor infiltrates at the paravertebral soft tissue (arrows) and into the kidney (open arrows) than PET/CT due to relatively low FDG uptake. (e) Microscopic photographs (upper: hematoxylin and eosin, lower: immunohistochemical stain for CD10, original magnification 20×) of the bone marrow biopsy specimen show paratrabecular infiltrates of atypical CD10 (+) small lymphocytes (diffuse infiltration), which is undetected by FDG-PET. Note “focal” ischial bone involvement and the biopsy tract (arrowheads)


Fig. 53.9
(a) Whole-body FDG-PET MIP, (b) axial PET and PET/CT, (c, d) DW images with b-values of 50 and 800 s/mm2 and their corresponding ADC maps in a 56-year-old patient with follicular lymphoma. Two renal cortical nodules show only mild FDG uptake (arrows on right and open arrows on left kidneys), while DW images with a b-value of 800 s/mm2 and ADC maps demonstrate excellent lesion-to-normal tissue contrast. Note mesenteric nodal/cecal involvement and renal parenchymal T2 shine-through effect on DW images with a b-value of 50 s/mm2. The patient was upstaged from Ann Arbor stages II to IV due to renal involvement
The most recent data showed that routine bone marrow has little or no therapeutic consequence for PET/CT-staged treatment-naive patients with Hodgkin lymphoma (HL) [28]. Bone marrow biopsy is more critical in non-Hodgkin lymphoma (NHL) than HL, and PET-positive bone/bone marrow findings should be confirmed by biopsy or MRI shall a change in treatment is planned [21].
53.4.2 Aggressive Transformation
Enhanced glucose uptake visualized by FDG-PET correlates with poor prognosis and higher metabolic potential in many tumor types [29]. It is believed that aggressive tumor cell with high proliferation rate shows high energy demand. Being able to quantify uptake values using most commonly the standardized uptake value (SUV), PET also provides a clinically important metabolic biopsy tool in a noninvasive way. It has been shown retrospectively in 97 patients with NHL that FDG uptake is lower in indolent than in aggressive lymphoma for both newly diagnosed and relapsed diseases [30] (Fig. 53.10). Although there was some overlap of the SUVs between indolent and aggressive disease, all cases of indolent lymphoma had an SUV equal to or less than 13 [30]. Results from later studies also corroborated this observation [31, 32]. Based on these reports, a cutoff of SUV at 14 is generally used to predict aggressive transformation and to redirect biopsy site in a patient with biopsy-proven indolent lymphoma but with high FDG uptake.
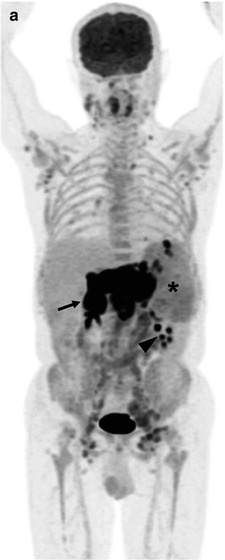
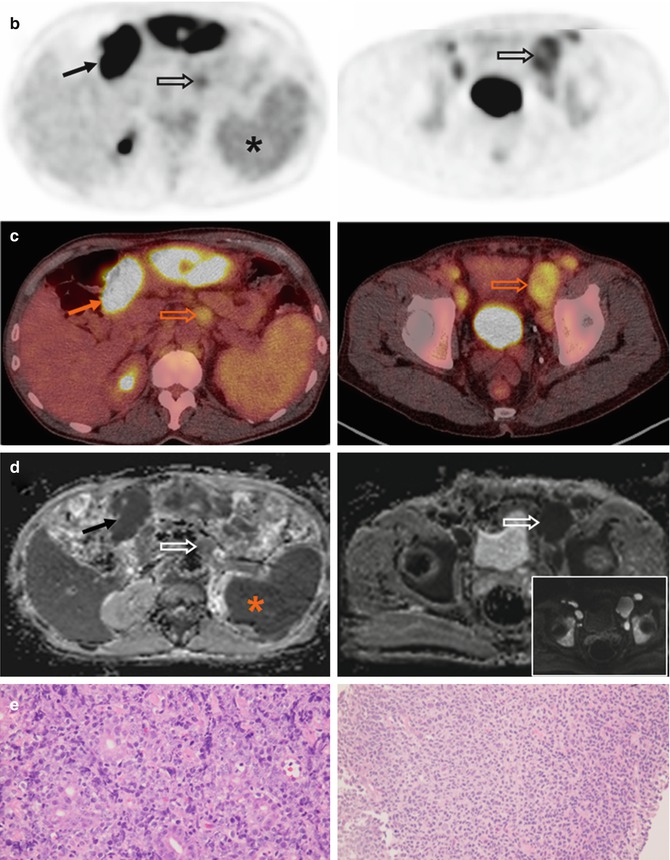


Fig. 53.10
(a) Whole-body FDG-PET MIP, (b) axial PET, (c) PET/CT, (d) ADC images at superior mesenteric root and bladder levels, and (e) microscopic photographs (hematoxylin and eosin, original magnification 40×) in a 63-year-old patient with concomitant DLBCL and follicular lymphoma. The gastric tumor shows intense FDG uptake (arrows), corresponding to the aggressive lymphoma component (e, left). Retroperitoneal and external iliac nodes (open arrows) show relatively low uptake, corresponding to the indolent component (e, right). The bone marrow and spleen have higher FDG uptake than liver and were both considered diffusely involved on PET. Since normal bone marrow and spleen already present with restricted diffusion, this can be suspected in case of markedly increased marrow signal intensity (inset in d: DW image with a b-value of 50 s/mm2) or splenomegaly. Note stasis of radioactivity in left renal calyces (arrowhead)
53.4.3 Response Assessment
53.4.3.1 End Treatment
In aggressive lymphomas, mainly the diffuse large B-cell lymphoma (DLBCL) and HL, FDG-PET has been integrated into the revised IWG criteria for treatment response assessment at the end of treatment [3, 4].
As mentioned earlier, PET information helps differentiating posttreatment fibrosis/necrosis from viable tumor more accurately than diagnostic CT does. PET increased the number of complete remission, allowing the elimination of complete remission unconfirmed (CRu) category [33] (Fig. 53.3). These new criteria incorporating PET have been validated by various groups and actively applied in ongoing therapeutic trials. Mediastinal blood pool activity is recommended as the reference background activity to define PET positivity for a residual mass ≥2 cm in greatest transverse diameter, regardless of its location. A smaller residual mass or a normal-sized lymph node (i.e., ≤1 × 1 cm in diameter) should be considered positive if its activity is above that of the surrounding background [4]. Specific criteria for defining PET positivity in the liver, spleen, lung, and bone marrow are also proposed. A recent prospective study also demonstrated that PET response assessment at the end of therapy was strongly predictive of outcome in 121 previously untreated patients with high-tumor burden follicular lymphoma [34].
53.4.3.2 Interim
Several studies have shown that treatment response assessed during treatment on “early” PET predicts patient outcome [35–37], which may help in patient stratification and therapeutic strategies. Early (or interim) PET is performed after one to four cycles of a six- to eight-cycle chemotherapy regimen and most commonly after two cycles nowadays. Again, the prognostic value of interim PET has been most extensively studied in DLBCL and HL. In DLBCL, patients with “negative” interim PET have superior complete response rate, event-free survival, and overall survival to those with residual abnormal uptake, irrespective of International Prognostic Index (IPI) or rituximab (anti-CD20 monoclonal antibody) target therapy [35]. More recent data confirmed its prognostic value in patients all treated with rituximab [38]. However, image interpretation based on visual analysis can be subjective and may be suboptimal for the interim PET studies since some patients with minimal residual uptake after only a few chemotherapy cycles still have favorable outcome [35, 39] (Fig. 53.11).
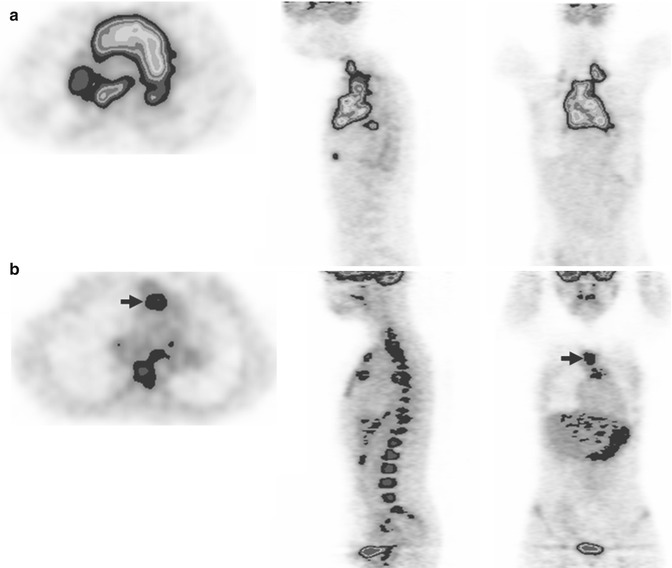

Fig. 53.11
Color-scaled 3-plane PET images in a 25-year-old patient with DLBCL before (a) and after treatment (b). Bulky mediastinal mass is noted at baseline, while only minimal residual uptake (intensity slightly higher than liver) is identified after two cycles of chemotherapy (arrows in b). The interim PET was considered positive based on visual analysis. However, there was over 80 % of the reduction of maximum standardized uptake value (delta SUVmax), and the patient remained free of disease with at least 3-year follow-up. Note diffusely increased bone marrow uptake commonly observed following chemotherapy
While visual assessment alone is considered adequate for interpreting PET findings as positive or negative when assessing response after completion of therapy [4], early PET interpretation may require semiquantitative analysis so that patients’ early chemosensitivity can be more objectively appreciated. Using 65.7 % as the cutoff for the reduction of maximum SUV (delta SUVmax) from baseline to two-cycle PET, the accuracy was 76 % with 2-year progression-free survival of 79 % versus 21 %, respectively, for those greater than and lower than the threshold [40] (Fig. 53.12). The positive predictive value of interim PET for the event (relapse or death) prediction was much improved, and 14 out of 34 PET-positive patients could have been classified as good responders correctly [40]. This post hoc response criterion has been externally validated later in a prospective phase II randomized trial [41].
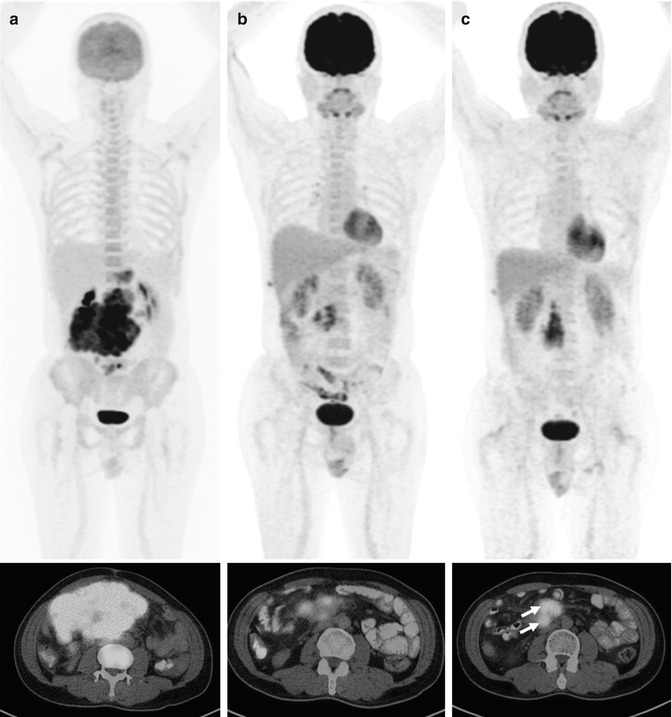

Fig. 53.12
Whole-body FDG-PET MIP and axial PET/CT images of a 51-year-old patient with DLBCL at three time points: (a) baseline, (b) after two cycles (interim), and (c) six cycles of chemotherapy (end treatment). At interim, the bulky mesenteric mass had much regression in volume; however, the delta SUVmax was 65.6 % (lower than the previously proposed optimal cutoff), and the persistent uptake foci progressed at the end of therapy (arrows)
In HL, patients with negative PET scan based on visual analysis after two cycles of chemotherapy also present with durable response and survival [42]. Interim PET results were shown to be the most important prognostic factor, more powerful than International Prognostic Score (IPS) in advanced-stage HL patients [43], potentially saving those in the good prognostic group from additional radiation therapy [44]. Although there is still no direct evidence that guiding therapy on the basis of interim PET findings improves lymphoma patients’ outcome, clinical trials are ongoing to evaluate the benefits of escalation and de-escalation treatment strategies [45]. Most importantly, the Deauville criteria have been proposed for the interpretation of interim PET in international validation studies [46, 47].
The Deauville criteria were designed as a semiquantitative visual analysis using a 5-point scale (Table 53.1) with the liver instead of mediastinal blood pool activity as the reference background, therefore reducing the false-positive rate [46, 48]. These criteria are commonly applied in the HL setting and are particularly useful in DLBCL studies when no baseline PET is available (unable to calculate the delta SUVmax). In addition, the Deauville scale eliminates the reference to the size of the residual mass, which prevents different readers to compare the same residual uptake to different backgrounds, mainly when a residual tumor of about 2 cm is assessed [46]. This simplifies the interpretation of interim PET and will probably be integrated in the next version of post-therapy criteria.
Table 53.1
Deauville 5-point scale
Score 1 | No residual uptake |
Score 2 | Uptake ≤ mediastinum |
Score 3 | Uptake > mediastinum but ≤liver |
Score 4 |