Gastric Carcinoma
Todd M. Blodgett, MD
Alex Ryan, MD
Hesham Amr, MD
Key Facts
Terminology
Adenocarcinoma arising from gastric mucosa
Imaging Findings
Hypermetabolic FDG activity in primary gastric tumor, regional lymph nodes (LN), peritoneum, distant metastases
Regional LN > 8 mm: Suspicious for metastatic disease
Peritoneal dissemination: Peritoneal caking, nodularity, beaded thickening, malignant ascites
No method currently available capable of staging N disease accurately enough to enable decision making based on the extent of surgical lymph node dissection
PET/CT for staging, recurrence, response to therapy
CECT: Focal gastric wall thickening or mass lesion with enhancement
PET sensitivity for primary tumor depends on extent
PET and CT similar sensitivity for primary (94% vs. 93%), PET higher specificity (92% vs. 62%)
Lymphadenopathy: PET insensitive for N1 disease (56%), equally sensitive for N2-3 disease as CT (78%)
Mucinous and signet-ring cell adenocarcinoma: Low FDG avidity 2° to high mucus, lack of glut-1 transporters
Top Differential Diagnoses
Gastric Ulcer
Gastrointestinal Stromal Tumor (GIST)
Gastric Lymphoma
Variable PET activity
Gastritis
Usually mild activity; nonfocal
Physiologic FDG Activity
TERMINOLOGY
Abbreviations and Synonyms
Gastric adenocarcinoma, early/advanced gastric carcinoma (EGC/AGC), gastric cancer, stomach cancer
Definitions
Adenocarcinoma arising from gastric mucosa
Malignant pathway: Gastritis → gastric atrophy → metaplasia → dysplasia → cancer
Most common primary tumor to metastasize to ovaries, usually bilaterally (Krukenberg tumors)
IMAGING FINDINGS
General Features
Best diagnostic clue
FDG PET
Hypermetabolic FDG activity in primary gastric tumor, regional lymph nodes (LN), peritoneum, distant metastases
CT
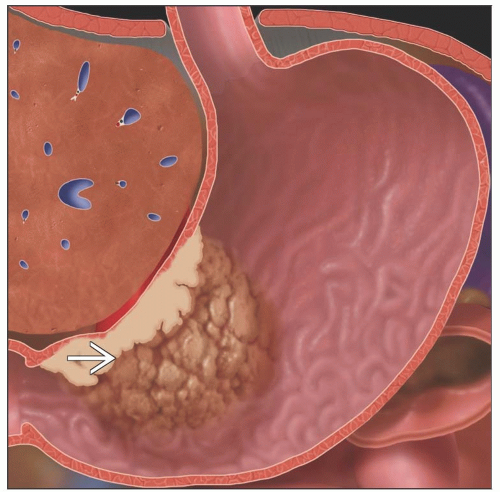
Polypoid mass ± ulceration
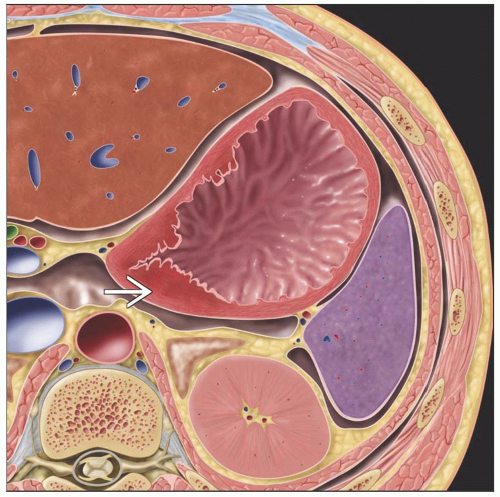
Focal wall thickening with mucosal irregularity, ulceration
Regional LN > 8 mm: Suspicious for metastatic disease
Peritoneal dissemination: Peritoneal caking, nodularity, beaded thickening, malignant ascites
Location
Fundus and cardia: 40%
Body: 30%
Antrum: 30%
Most likely sites of recurrence post-gastrectomy: Gastric bed, peritoneal dissemination, liver
Large proportion of non-FDG-avid, non-intestinal subtype tumors found in distal 2/3 of stomach
Tumor commonly invades directly into
Pancreas via lesser sac
Transverse colon via gastrocolic ligament
Liver via gastrohepatic ligament
60% of carcinoma of cardia will spread to distal esophagus
5-20% of antral carcinoma will involve duodenum
Size: Often very large before tumor becomes symptomatic
Morphology
Type I: Elevated, protrude > 5 mm into lumen
Type II: Superficial lesions that are elevated (IIa), flat (IIb), depressed (IIc)
Type III: Early gastric cancers that are shallow, irregular ulcers surrounded by nodular, clubbed mucosal folds
Imaging Recommendations
Best imaging tool
PET/CT for staging, recurrence, response to therapy
No modality is currently sensitive enough to confidently guide surgical planning
CECT to determine whether tumor is resectable
Barium X-ray screening is effective but has low sensitivity
Side effects include constipation and mis-swallowing of barium into trachea
Protocol advice
PET sensitivity for recurrent disease postgastrectomy may be ↑ by water ingestion (˜ 300 cc) to distend remnant stomach
FDG uptake secondary to insulin release associated with food intake can be avoided by feeding at 120 minute time point after FDG administration
CT evaluation also aided by negative contrast ingestion (water or gas)
CT Findings
General features
Mucosal irregularity
Enhancing focal gastric wall thickening
Gastric distention important for accurate assessment
Enhancing mass lesion
Normal gastroesophageal junction may be mistaken for mass
Polypoid mass ± ulceration
Gas-filled ulcer crater within mass
Tumor depth difficult to assess
Primary by type
Infiltrating type: Loss of normal rugal folds over thickened wall
Scirrhous type: Thickened wall with marked contrast enhancement
Mucinous type: Wall thickening and calcification with decreased attenuation due to mucin content
Cardia tumor: Lobulated mass with irregular soft tissue thickening
Extension/metastasis
CT can delineate fat pad between tumor and organ to determine resectability
May normally be absent between stomach and left lobe of liver
Inflammation can obscure fat plane between tumor and pancreas
Cachexia results in loss of fat planes, producing potential false positive for organ invasion
Can often detect peritoneal carcinomatosis
Valuable for avoiding futile laparotomies
Tiny deposits may be overlooked
Extension into perigastric fat appears as wisp-like perigastric soft tissue stranding
Lymph nodes
Subcentimeter lymph nodes may harbor malignancy
Enlarged lymph nodes may be nonmalignant (inflammation, infection)
Perigastric nodes are best visualized when stomach is fully distended
Nuclear Medicine Findings
General
False positives
Normal stomach uptake
Gastritis
Inflammatory regional lymph nodes
Cholecystitis can produce false positives in liver
Water or food ingestion may decrease false positive from 31% to 8%
Initial diagnosis
FDG PET sensitivity for primary tumor
Early GC: 47%
Advanced GC: 98%
In general, better sensitivity for more advanced disease
PET and CT similar sensitivity for primary (94% vs. 93%)
PET has higher specificity (92% vs. 62%)
In general, well-differentiated gastric adenocarcinomas tend to take up less FDG than poorly differentiated ones
Exceptions
Poorly differentiated tubular adenocarcinomas show an especially wide spectrum of FDG uptake, from low to intense
Mucinous and signet-ring cell adenocarcinoma: Low FDG avidity 2° to high mucus, lack of glut-1 transporters
Staging
Pre-treatment staging is essential to determine potential curability and to plan optimal therapy
Lymphadenopathy
PET and CT have similar sensitivity for detection of local and distant lymphadenopathy
Overall lymph node evaluation: PET less sensitive than CT (56% vs. 78%) but more specific (92% vs. 62%)
PET alone has low sensitivity for N1 disease (56%)
N1 insensitivity less important because all patients with AGC will undergo at least D1 dissection
PET and CT have high specificity for N1 disease
Positive findings may change endoscopic mucosal resection to more aggressive surgical approach in patients with EGC
Almost all PET-positive lymph nodes prove to be involved in patients with AGC
PET and CT equally sensitive for N2-3 disease (about 80%)
N2/N3 status determination important: Can change extent of lymph node dissection and, in the case of N3 disease, curative potential
Peritoneal dissemination: PET has low sensitivity and CT low specificity, but similar accuracy overall
FDG avidity of primary tumor predictive of long-term survival in patients undergoing complete resection of primary
Response to therapy
Post-therapy change in tumor SUV predicts both response to therapy and long-term survival with 77% sensitivity, 86% specificity
Decreased tumor uptake by > 35% of baseline allowed accurate prediction of response 14 days after initiation of cisplatin-based polychemotherapy
Overall accuracy of 83%
1/3 of patients initially have insufficient FDG uptake for quantification
Initially low FDG uptake probably correlates with tumors in which chemotherapy has limited effectiveness
Other radiotracers
18F-fluorothymidine sensitive for locally advanced gastric cancers with improved detection of tumors with signet ring cells or mucinous contents
DIFFERENTIAL DIAGNOSIS
Gastric Ulcer
Benign ulcer
Crater margin sharply defined and smooth en face, symmetric, confluent with healthy mucosa, mucosal folds radiate from ulcer edge
Most located in lesser curve or posterior wall of antrum, body of stomach
Gastrointestinal Stromal Tumor (GIST)
Well-demarcated, spherical, intramural masses that arise from muscularis propria; often project exophytically, intraluminally
May have overlying mucosal ulceration
Larger GISTs often outgrow vascular supply → necrosis and hemorrhage
Gastric Lymphoma
Usually correlative gastric wall thickening
May be diffuse throughout stomach
Variable PET activity
Gastritis
Usually mild to moderate FDG activity
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree