Chapter 3 Gastrointestinal tract
Methods of imaging the gastrointestinal tract
Ambrosini R., Barchiesi A., Di Mizio V., et al. Inflammatory chronic disease of the colon: how to image. Eur. J. Radiol.. 2007;61(3):442-448.
Brochwicz-Lewinski M.J., Paterson-Brown S., Murchison J.T. Small bowel obstruction the water soluble follow-through revisited. Clin. Radiol.. 2003;58(5):393-397.
Gasparaitis A.E., MacEneaney P. Enteroclysis and computed tomography enteroclysis. Gastroenterol. Clin. North Am.. 2002;31(3):715-730.
Introduction to Contrast Media
BARIUM
Barium suspension is made up from pure barium sulphate. (Barium carbonate is poisonous.) The particles of barium must be small (0.1–3 µm), since this makes them more stable in suspension. A non-ionic suspension medium is used, for otherwise the barium particles would aggregate into clumps. The resulting solution has a pH of 5.3, which makes it stable in gastric acid.
There are many varieties of barium suspensions in use. Exact formulations are secret. In most situations the preparation will be diluted with water to give a lower density (Table 3.1).
Table 3.1 Barium suspensions and dilutions with water to give a lower density
| Proprietary name | Density (w/v) – use |
|---|---|
| Baritop 100 | 100% – all parts gastrointestinal tract |
| EPI-C | 150% – large bowel |
| E-Z-Cat | 1–2% – computed tomography of gastrointestinal tract |
| E-Z HD | 250% – oesophagus, stomach and duodenum |
| E-Z Paque | 100% – small intestine |
| Micropaque DC | 100% – oesophagus, stomach and duodenum |
| Micropaque liquid | 100% – small and large bowel |
| Micropaque powder | 76% – small and large bowel |
| Polibar | 115% – large bowel |
| Polibar rapid | 100% – large bowel |
Examinations of different parts of the gastrointestinal tract require barium preparations with differing properties:
Advantages
Disadvantages
Complications
For further complications (e.g. constipation and impaction), see the specific procedure involved.
Water-Soluble Contrast Agents
Indications
GASES
1 Holemans J.A., Matson M.B., Hughes J.A., et al. A comparison of air, carbon dioxide and air/carbon dioxide mixture as insufflation agents for double contrast barium enema. Eur. Radiol.. 1998;8:274-276.
Pharmacological Agents
Hyoscine-N-butyl bromide (Buscopan)
This is an antimuscarinic agent and, therefore, inhibits both intestinal motility and gastric secretion. It is not recommended in children.
Glucagon
This polypeptide hormone produced by the alpha cells of the islets of Langerhans in the pancreas has a predominantly hyperglycaemic effect but also causes smooth muscle relaxation.
Advantages
Disadvantages
Contraindications
1 British National Formulary. 2007;54.
General points
CONTRAST SWALLOW
Indications – suspected oesophageal pathology
Contrast medium
Equipment
Rapid serial radiography (6 frames per s) or video recording may be required for assessment of the laryngopharynx and upper oesophagus during deglutition.
Patient preparation
None (but as for barium meal if the stomach is also to be examined – see p. 57).
Preliminary film
A control film is advised prior to a water-soluble study if perforation is suspected.
Technique
Modification of technique
To demonstrate a tracheo-oesophageal fistula in infants, a ‘pull back’ nasogastric tube oeosophogram may be performed. A nasogastric tube is introduced to the level of the mid-oesophagus, and the contrast agent (barium or LOCM) is syringed in to distend the oesophagus. This will force the contrast medium through any small fistula which may be present. It is important to take radiographs in the lateral projection during simultaneous injection of the contrast medium and withdrawal of the tube. Although some authors recommend that the infant be examined in the prone position whilst lying on the footstep of a vertical tilting table, satisfactory results are possible with children on their side on a horizontal table. It is important to watch for any possibility of aspiration into the airway from overspill. Overspill may lead to the incorrect diagnosis of tracheo-oesophageal fistula if it is not possible to determine whether contrast medium in the bronchi is due to a small fistula which is difficult to see or to aspiration.
Recently, it has been proposed that pull-back studies are not necessary in the majority of children, as tracheo-oesophageal fistulas can usually be demonstrated on standard contrast swallow examination, providing the oesophagus is distended well with contrast media.1 Pull-back studies are still necessary for intubated patients, or those who are at high risk of aspiration. It is important to remember that fistulas are usually quite high, and the orifice can be occluded by an endotracheal tube. This can prevent the fistula being opacified. This can be rectified by altering the patients position, or slightly withdrawing the ET tube.
1 Laffan E.E., Daneman A., Ein S.H., et al. Tracheoesophageal fistula without esophageal atresia: are pull-back tube esophagograms needed for diagnosis. Pediatr. Radiol.. 2006;36:1141-1147.
Barium Meal
Methods
Indications
Patient preparation
Technique
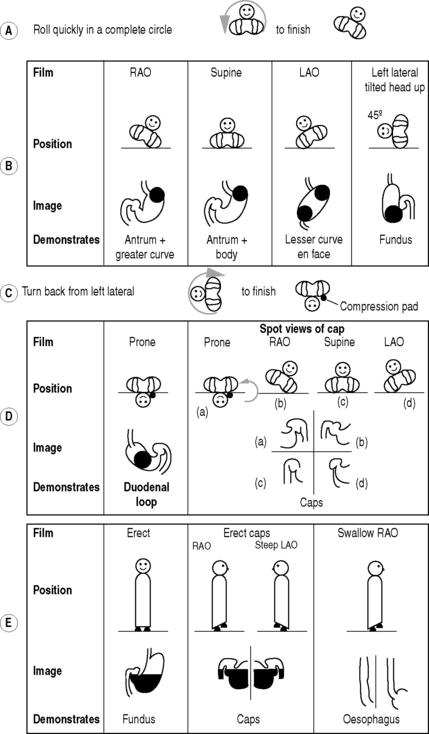
The double contrast method (Fig. 3.1):
Films
There is a great variation in views recommended, and the following is only the scheme used in our departments. In some departments fewer films are taken to reduce the cost and radiation dose:
From the left lateral position the patient returns to a supine position and then rolls onto the left side and over into a prone position. This sequence of movements is required to avoid barium flooding into the duodenal loop, which would occur if the patient were to roll onto the right side to achieve a prone position.
An additional view to demonstrate the anterior wall of the duodenal loop may be taken in an RAO position.
Modification of technique for young children
The main indication will be to identify a cause for vomiting. The examination is modified to identify the three major causes of vomiting – gastro-oesophageal reflux, pyloric obstruction and malrotation, and it is essential that the position of the duodeno-jejunal flexure is demonstrated:
In newborn infants with upper intestinal obstruction, e.g. duodenal atresia, the diagnosis may be confirmed if 20 ml of air is injected down the nasogastric tube (which will almost certainly have already been introduced by the medical staff). If the diagnosis remains in doubt, it can be replaced by a positive contrast agent (dilute barium or LOCM if the risk of aspiration is high).
Aftercare
Complications
N.B. It must be emphasized that there are many variations in technique, according to individual preference, and that the best way of becoming familiar with the sequence of positioning is actually to perform the procedure oneself.
BARIUM FOLLOW-THROUGH
Methods
Indications
Contraindications
Contrast medium
E-Z Paque 100% w/v 300 ml usually given in 10–15-min increments, although some radiologists give the full 300 ml at once. The transit time through the small bowel has been shown to be reduced by the addition of 10 ml of Gastrografin to the barium. In children, 3–4 ml kg−1 is a suitable volume.
In situations where barium is contraindicated, non-ionic water-soluble solutions have been shown to be a satisfactory alternative.1
Technique
The aim is to deliver a single column of barium into the small bowel. This is achieved by laying the patient on his right side after the barium has been ingested. Metoclopramide enhances the rate of gastric emptying. If the transit time through the small bowel is found to be slow, the addition of an osmotic water-soluble contrast agent may help to speed it up. If a follow-through examination is combined with a barium meal, glucagon is used for the duodenal cap views rather than Buscopan because it has a short length of action and does not interfere with the small-bowel transit time.
Films
Additional films
1 Jobling C., Halligan S., Bartram C. The use of water-soluble contrast agents for small bowel follow-through examinations. Eur. Radiol.. 1999;9:706-710.
Ha H.K., Shin J.H., Rha S.E., et al. Modified small bowel follow through: use of methylcellulose to improve bowel transradiance and prepare barium suspension. Radiology. 1999;211:197-201.
Summers D.S., Roger M.D., Allan P.L., et al. Accelerating the transit time of barium sulphate suspensions in small bowel examinations. Eur. J. Radiol.. 2007;62(1):122-125.
Small-Bowel Enema
Advantage
This procedure gives better visualization of the proximal small bowel than that achieved by a barium follow-through because rapid infusion of a large, continuous column of contrast medium directly into the jejunum provides better distension of the proximal small bowel. This is less effective in the ileum.
Disadvantages
Indications and Contraindications
These are the same as for a barium follow-through. In some departments it is only performed in the case of an equivocal follow-through.
Contrast medium
It may be difficult to obtain good distension and double-contrast effect of the distal small bowel and terminal ileum.
Equipment
A choice of tubes is available:
Patient preparation
Immediately before the examination the pharynx is anaesthetized with lidocaine spray.
Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 = Barium
= Barium