Fig. 10.1
a Normal pylorus b Hypertrophic pyloric stenosis—4-week-old male who presented with forceful nonbilious emesis. Pyloric muscle measured 4 mm (triangles), pyloric channel measured 17 mm (circles)
Malrotation and Volvulus
Malrotation (incidence 1:6000) is caused by failure of the intestine to undergo the normal 270° counterclockwise rotation around the SMA during embryologic development. Midgut volvulus results from torsion of the bowel around an abnormally narrow mesentery that results from malrotation and improper fixation of the bowel. It can result in catastrophic necrosis and loss of a majority of the intestine if not recognized and treated promptly with surgical derotation. The typical patient is a previously healthy term infant in the first month of life who presents with sudden onset bilious emesis. Traditionally, the diagnosis is made with abdominal radiograph and upper GI studies [15].
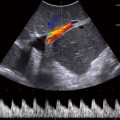
Currently, ultrasound is utilized in some centers when the diagnosis of malrotation or acute midgut volvulus is equivocal. Color Doppler is employed to demonstrate the “whirlpool” sign, a swirling of the blood vessels caused by the twisting of the mesentery (Fig. 10.2), or an inversion of the location of the SMA and superior mesenteric vein [16]. Malrotation is often accompanied by dilation of the duodenum. There is a potential role for diagnosing malrotation based on the position of the duodenum relative to the SMA and aorta. Yousefzadeh and colleagues demonstrated the feasibility and validity of this technique in 33 neonates [17]. If normal anatomy is seen on abdominal ultrasound, additional studies and radiation exposure may be avoided.
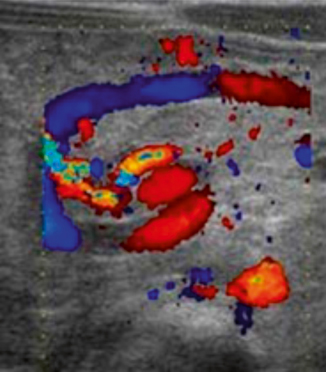

Fig. 10.2
Malrotation with volvulus—color Doppler ultrasound demonstrates “whirlpool” sign
Intussusception
Intussusception , or intestinal invagination is the process by which one segment of proximal bowel telescopes onto the adjacent distal segment. It can result in obstruction, abdominal pain, and ultimately ischemic bowel due to compromised blood flow. Abdominal ultrasound, first described for the evaluation of this entity in 1977, is the primary imaging modality for diagnosis of intussusception today. The hallmark sign is the “target” or “doughnut” sign (Fig. 10.3), an image seen in the transverse plain that demonstrates bowel wall and mesenteric fat within the intussusceptum forming rings of high and low echogenicity. Another characteristic finding is the “pseudokidney” sign (Fig. 10.4), an image seen in the longitudinal plain that demonstrates the edema in the walls of the intussusceptum trapped inside the intussuscipiens [18].
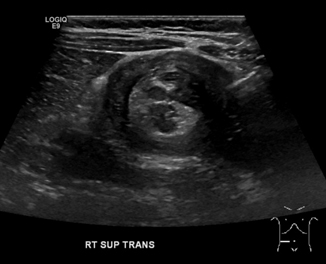
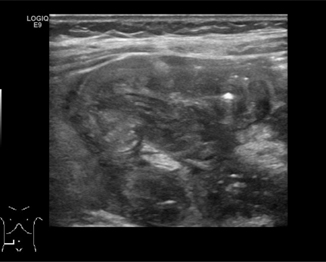

Fig. 10.3
Intussusception—5-month-old male who presented with fussiness, drawing legs up to chest, and blood per rectum. Ultrasound showed ileocolic intussusception, “target” sign

Fig. 10.4
Intussusception—5-month-old male who presented with fussiness, drawing legs up to chest, and blood per rectum. Ultrasound showed ileocolic intussusception, “pseudokidney” sign
Intussusception can be reduced with normal saline hydrostatic enema under ultrasound guidance. Many centers utilize air enema and fluoroscopy to confirm reduction. However, ultrasound can visualize the reduction of the ileo-ileocolic intussusception as well as the development of any pneumoperitoneum should perforation complicate the procedure [19]. Inability to complete the reduction under ultrasound guidance should prompt an attempt at conventional air/contrast enema reduction.
Intestinal Atresia
Intestinal atresias are congenital obstructions of the lumen of the duodenum, jejunum, or ileum thought to be due to vascular accidents in utero. They represent a relatively common etiology of neonatal bowel obstruction (incidence 1.3–2.8:10,000) and are frequently diagnosed on antenatal ultrasound. Duodenal atresia is reliably diagnosed prenatally through recognition of the classic “double bubble” sign, but identifying jejunal and ileal atresias can be more challenging with lower and more variable rates of prenatal diagnosis. Imaging characteristics including polyhydramnios and dilated bowel may not be present at the time of ultrasound [20].
For neonates who carry a diagnosis of suspected atresia, many centers utilize abdominal ultrasound as confirmatory testing and to evaluate for additional anomalies [21]. Characteristics of duodenal atresia include massive dilation of the stomach and normal pylorus with decompressed distal intestine (Fig. 10.5).
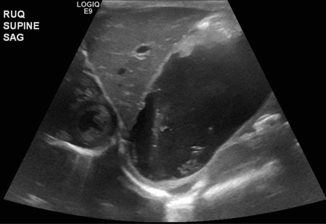

Fig. 10.5
Duodenal atresia—5-day-old male with emesis and no passage of stool. Ultrasound showed hugely distended stomach containing anechoic fluid, anatomically and functionally normal pylorus that opened well to allow passage of a small amount of gastric contents into the duodenum seen during the scanning process
Characteristics of intestinal atresia on ultrasound may include dilated loops of proximal bowel and decompressed distal bowel segments; direct visualization of the atretic segment is also possible (Figs. 10.6 and 10.7). In previously healthy neonates who present with symptoms of bowel obstruction, a variety of imaging techniques are employed for evaluation including plain radiograph, upper GI series, contrast enema, and ultrasound, among others. Ultrasound has the advantage of not only demonstrating the intestinal atresia but also ruling out ascites , perforation, abdominal masses, or other etiology of obstruction.
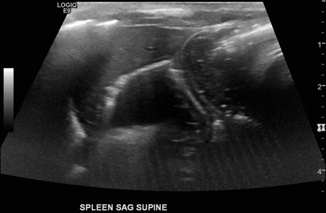
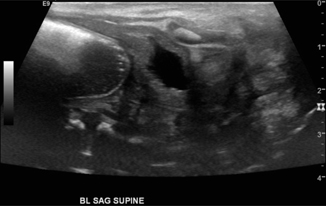

Fig. 10.6
Jejunal atresia: 2-day-old male with intestinal atresia type 4, total of 15 atretic segments. Ultrasound shows multiple dilated loops of bowel

Fig. 10.7
Jejunal atresia: 2-day-old male with intestinal atresia type 4, total of 15 atretic segments. Ultrasound shows multiple dilated loops of bowel
Meckel Diverticulum
Meckel diverticulae represent the most common anomaly of the GI tract with prevalence in the general population from 1 to 4 %. The clinical presentation can be variable and includes GI bleed, obstruction, diverticulitis, perforation, and volvulus [22]. An inflamed Meckel’s diverticulum functions commonly as the lead point of a nonreducible intussusception, then diagnosed and resected in the operating room. Abdominal ultrasound is frequently obtained in the evaluation of pediatric abdominal pain, particularly when patients present with symptoms that are nonspecific. A Meckel’s diverticulum appears on ultrasound as a long tubular structure and can mimic a duplication cyst. It can also be confused for an acutely inflamed appendix, as in the case of Meckel’s diverticulitis (Fig. 10.8). If a diverticulum is the lead point causing intussusception , ultrasound will reveal a “double-target sign,” a target-shaped mass of rings of high and low echogenicity with an additional central area of hyperechogenicity.
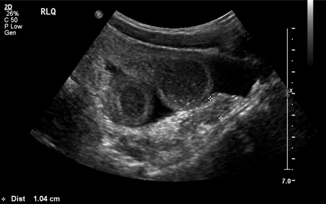

Fig. 10.8
9-year-old male with Meckel’s diverticulitis—distended, blind-ending tubular structure in right lower quadrant consistent with the appendix which measures 1 cm in diameter. Several loops of dilated bowel with wall thickening are also seen. Additionally, free fluid is identified in the right lower quadrant and in Morison’s pouch
Abdominal Cysts
Enteral Duplication Cyst
Intestinal duplication cysts are uncommon congenital anomalies (incidence 1–2:10,000) that can be seen throughout the GI tract (Figs. 10.9 and 10.10), but are most commonly found in the ileum. Most are diagnosed before age 2 after the patient presents with obstructive symptoms, vague pain or constipation, or rarely with GI bleeding or malignant degeneration. The treatment is surgical excision of the lesion. Ultrasound diagnosis is made by the “double wall” appearance (Fig. 10.11)—a hyperechoic edge surrounding the mucosal wall inside and a hypoechoic wall surrounding the smooth muscular layer outside, which are continuous with the corresponding structures of the adjacent bowel [23].
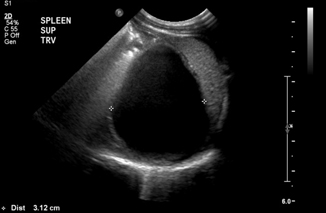
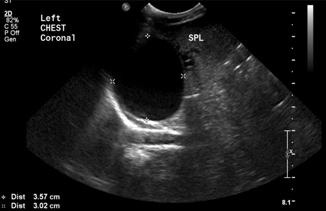
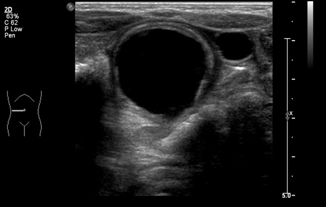

Fig. 10.9
Gastric duplication cyst at the level of the fundus, adjacent to the spleen—abdominal view

Fig. 10.10
Gastric duplication cyst at the level of the fundus, adjacent to the spleen—transthoracic view

Fig. 10.11
Ileal duplication cyst with “double wall” appearance
Mesenteric Cysts
Mesenteric cysts are a rare anomaly (incidence 1:100,000) with only 25–30 % of these diagnosed in children [24]. They can be found in small or large bowel mesentery after patients present with abdominal pain and undergo imaging to rule out other presumed pathology like appendicitis. Ultrasound evaluation is sensitive and specific, and can be used for diagnosis as well as to follow lesions of uncertain etiology. Imaging characteristics include a multiloculated septated cystic mass and ascites may or may not be present (Fig. 10.12). Sequential images may show increased size, increased fluid echogenicity, and thickening or multiplication of septae [25]. Surgical excision is the treatment of choice; pathology typically reveals benign tissue though complications may arise from cyst growth over time [26].
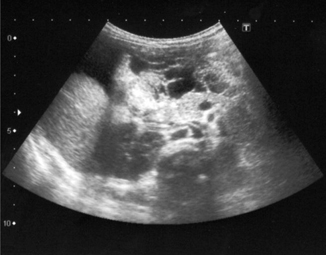

Fig. 10.12
Mesenteric cyst—multiloculated, septated cystic intra-abdominal mass
Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is a multifactorial disease that leads to disruption of intestinal integrity followed ultimately by bowel necrosis and bacterial translocation. Birth weight is inversely associated with incidence (1:1000) and mortality (15–30 %). Younger gestational age is also a predictor of poor outcome. The clinical presentation of NEC includes feeding intolerance, abdominal distention, and often bloody stool [27].
Currently, abdominal radiograph remains the preferred modality of choice for diagnosis and classification of NEC. However, abdominal ultrasound is increasingly being applied in NEC diagnosis and management for the advantages it offers over standard radiographs. Ultrasound permits visualization of bowel wall thickness and echogenicity, peristalsis, free fluid, and bowel wall perfusion [28]; this may allow for earlier diagnosis of bowel compromise and expedition of needed surgical intervention when bowel is threatened, but before actual perforation or frank necrosis occurs.
Pneumatosis intestinalis (PI) has been used as a marker for the condition. Most often observed in the ileum and colon, the finding is almost pathognomonic for NEC, though its presence ranges between 13 and 100 % of cases, depending on the study examined. Ultrasound has proven superior in the early diagnosis of PI. In a study of 40 neonates with Bell stage I NEC, ultrasound located PI in all 40, despite the lack of such findings on abdominal radiographs, demonstrating the value of ultrasound in making an early, definitive diagnosis [29]. However, for patients in ≥ Bell stage II NEC, abdominal radiographs have been shown, in some studies, to be superior in identifying pneumatosis. Given this variation, more studies are needed to clarify the role of ultrasound in diagnosing PI in neonates with NEC .
The protocol for ultrasound of the abdomen for NEC has been well described by Faingold et al. [30] and includes evaluation of the intestine for increased wall echogenicity, wall thickening (greater than 2.7 mm) suggestive of edema, wall thinning (less than 1.0 mm) suggestive of ischemia, intramural gas (PI; Fig. 10.13), bowel wall perfusion on color Doppler, and bowel peristalsis. The peritoneal cavity of the abdomen and pelvis is then evaluated for free-fluid or discrete-fluid collections, and the liver and portal venous system are evaluated for portal venous gas (Fig. 10.14). The splanchnic and hepatic circulation should then be assessed via Doppler for flow and perfusion. The sensitivity, specificity, positive predictive value, and negative predictive values of sonography for the detection of bowel necrosis were reported to be 100, 95, 80, and 100 %, respectively [31].
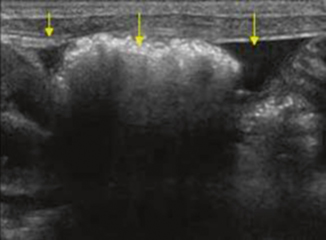
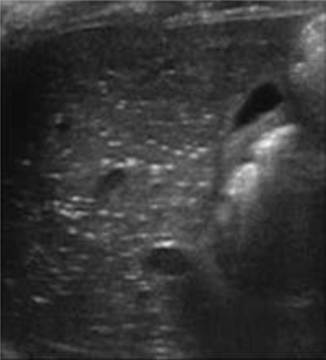

Fig. 10.13
Necrotizing enterocolitis—intramural gas

Fig. 10.14
Necrotizing enterocolitis—portal venous gas
Appendicitis
Ultrasound is a reliable tool in the workup of pediatric abdominal pain and diagnosis of acute appendicitis (sensitivity 72.5 % (95 % CI = 58.8–86.3 %) and specificity 97.0 % (95 % CI = 96.2–97.9 %)) [32, 33]. It is now the standard of care in pediatric centers to obtain ultrasound imaging as the first-line modality with selective use of CT scan in some cases. This demonstrates a change in practice that has decreased the burden of ionizing radiation in pediatric patients without an increase in the negative appendectomy or missed appendectomy rates [34, 35].
The sonographic criteria to diagnose acute appendicitis in children include visualizing a blind-ending tubular structure that is noncompressible (Fig. 10.15) and lacks peristalsis, with an appendicular diameter greater than 6 mm and/or a wall thickness greater than 2.0 mm.
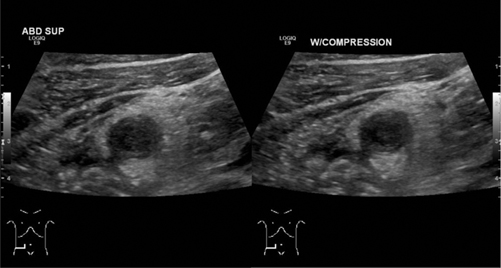

Fig. 10.15
Appendicitis—sagittal view of noncompressible enlarged appendix
Other signs to support the diagnosis of appendicitis include the lack of air in the appendiceal lumen, periappendiceal fat stranding, presence of appendicolith, complex right lower quadrant mass, enlarged mesenteric lymph nodes, and presence of free fluid [36] (Fig. 10.16). Recent evidence suggests that using a cutoff point of 7.0 mm appendicular diameter and 1.7 mm wall thickness [37] may be more predictive of acute appendicitis in pediatric patients.
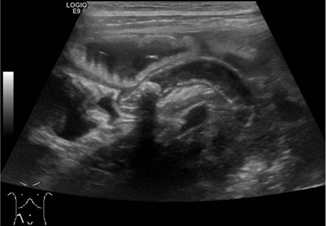

Fig. 10.16
Appendicitis—enlarged tubular blind-ending structure in right lower quadrant with fecalith visible at the base, longitudinal view
Ultrasound is operator dependent and is limited by the lie of the appendix. In up to one third of the cases, the appendix may be nonvisualized on ultrasound imaging with the patient in the traditional supine position. Some protocols recommend turning the patient to left posterior oblique position in an attempt to identify a potentially retrocecal appendix [38]. In the absence of a leukocytosis, patients with a nonvisualized appendix can be safely observed without the immediate need for additional imaging [39].
Anorectal Malformations
Anorectal malformations are a complex group of congenital anomalies with an incidence of approximately 1:5000 births. The most common presentation in males is imperforate anus (anal atresia) with a rectourethral fistula, and in females imperforate anus with a rectovestibular fistula [39]. Some patients are diagnosed antenatally after visualizing dilated distal bowel or rectum on obstetric ultrasound [40, 41], though the technical difficulty in making that diagnosis means that most patients are identified by physical examination on the first day of life. Ultrasound plays many roles in the care of patients with anorectal malformations, including diagnosis and preoperative planning, intraoperative guidance, and postoperative assessment.
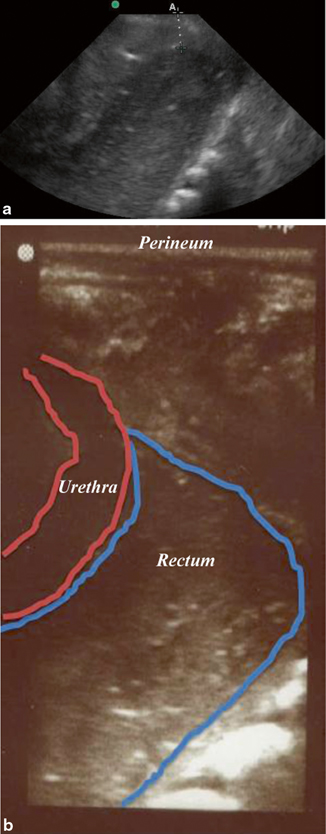
For children with imperforate anus, early diagnosis and clarification of the patient’s anatomy is critical in planning the appropriate surgical intervention. The distance from bowel to skin is a determining factor in the type of procedure that is indicated: for a low defect (< 1 cm bowel-skin distance), an anoplasty typically can be done without a colostomy; but for an intermediate or high defect (> 1 cm bowel-skin distance), a decompressive colostomy may be created before posterior sagittal anorectoplasty described by Pena [42]. Both radiographs (invertogram and prone cross-table lateral views) and ultrasound can be utilized to obtain this information. Transperineal ultrasound is feasible and valid for determining bowel-skin distance with sensitivity 100 % and specificity 86 % with an error in distance measurement of 0.12 cm (± 0.33) [43, 44] (Fig. 10.17).
Get Clinical Tree app for offline access

Fig. 10.17
a and b Imperforate anus—a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree