Abstract
Gastroschisis is a result of a full-thickness paraumbilical (usually right-sided) defect that allows herniation of free-floating fetal bowel into the amniotic cavity. The worldwide incidence of gastroschisis is increasing, and young mothers experience higher rates of gastroschisis. Approximately 10% of cases of gastroschisis have additional unrelated malformations, but it is rarely associated with aneuploidy (<2% of cases); cases with aneuploidy are frequently associated with additional fetal malformations. Several hypotheses for etiology exist; the most recent hypothesis describes a failure of the vitelline structures to be incorporated into the umbilical stalk. Prenatal detections rates are high (90-87%), and suspicion for the diagnosis can be raised when maternal serum alpha fetal protein levels are increased. The characteristic US appearance is of free-floating loops of fetal bowel in the amniotic cavity. The bowel is typically described as having a “cauliflower” appearance. Uncommonly, herniation of additional fetal organs is present. The differential diagnosis includes omphalocele, ruptured omphalocele, limb–body wall complex, bladder exstrophy, cloacal exstrophy, ectopia cordis, pentalogy of Cantrell, umbilical cord cysts, and urachal abnormalities. No prenatal therapy is available at the present time. Postnatal therapy includes primary closure, use of a Silastic spring-loaded silo, or staged closure. Overall survival rate is greater than 90% in developed countries. Long-term complications include bowel dysmotility, short gut syndrome, and complications from long-term total parenteral nutrition, including liver failure.
Keywords
gastroschisis, abdominal wall defect, ultrasound findings, prenatal diagnosis, obstetrical management
Introduction
Gastroschisis, a full thickness paraumbilical defect in the abdominal wall that results in herniation of the fetal midgut, has been considered an entity embryologically distinct from omphalocele since the mid-1950s. The widespread availability of prenatal ultrasound (US) and of maternal serum alpha-fetoprotein screening allows for routine antepartum diagnosis of gastroschisis with high accuracy. For reasons not well defined, the prevalence of gastroschisis has continued to increase both in the United States and internationally. The defect is usually isolated, without chromosomal abnormalities, but it carries a significant mortality rate of 5% to 10%. Infants with gastroschisis are more likely to be born prematurely and to be affected by intrauterine growth restriction. Bowel atresia and damage to the bowel secondary to persistent intrauterine contact with amniotic fluid is also implicated in neonatal morbidity and mortality. Because serial prenatal surveillance of affected fetuses is possible and the short-term and long-term neonatal sequelae are recognized, many practitioners have studied how to optimize fetal maturity while minimizing ongoing damage to the fetal bowel.
Disease
Definition
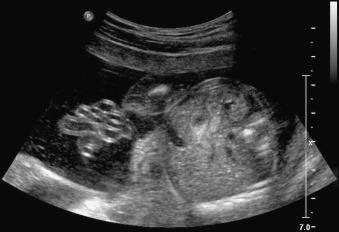
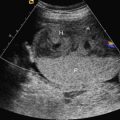
Gastroschisis (Greek for “abdominal cleft”) is a full-thickness paraumbilical defect in the abdominal wall. In most cases, the defect lies to the right of a normally inserted umbilical cord. Fetal bowel herniates through this defect and can be seen floating freely in the amniotic fluid ( Fig. 20.1 and ). The defect is usually small (<4 cm), and, by definition, there is no peritoneal membrane covering the herniated abdominal contents ( Fig. 20.2 ). Although it is possible to see herniation of additional organs, such as the stomach, liver, spleen, or genitourinary tract, this is less common. The diagnosis is easily made with US scanning starting at the end of the first trimester.
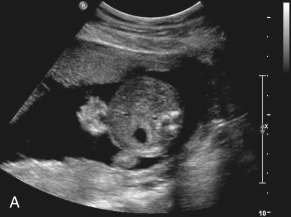
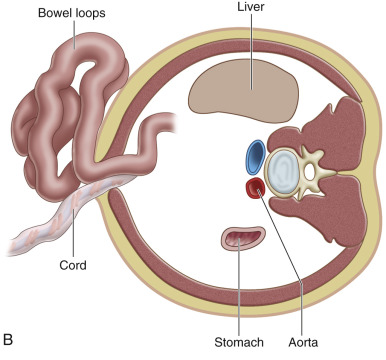
In contrast to omphalocele, the loops of exposed bowel are not protected from the amniotic fluid by the peritoneum. In addition, the blood supply to the herniated gut can be disrupted if there is kinking or compression of the bowel as it passes through the abdominal wall defect. These mechanisms can lead to an array of fetal and neonatal complications, including volvulus, bowel atresia, stenosis, or necrosis, hypoperistalsis, and short gut syndrome.
Prevalence and Epidemiology
The incidence of gastroschisis has been increasing worldwide for the past 2 to 3 decades. Reported rates of gastroschisis have increased over the past 25 years from 0.1 : 10,000 to 1.0 : 10,000 live births in developed countries, and from 3.0 : 10,000 to 5.0 : 10,000 births in underdeveloped countries. The variation in incidence is attributed to geographic distribution and maternal age. Studies have consistently reported a several-fold higher rate of gastroschisis in pregnancies of women younger than 20 years old. Mastroiacovo et al. evaluated the data of 25 registries of members of the International Clearinghouse for Birth Defects Surveillance and Research with 7 years of data and found 14 registries with a significantly increasing temporal trend of gastroschisis. No similar trend was observed in 36 other malformations analyzed. The increasing trend is worldwide but not universal. Increased incidence is seen in Japan; Australia; North, Central, and South America; and North-Central Europe. United States Centers for Disease Control and Prevention (CDC) data from 1995 to 2012 revealed a 30% increase in the prevalence of gastroschisis when comparing the time periods 1995–2005 and 2006–2012, with the prevalence increasing from 3.6 : 10,000 to 4.9 : 10,000 live births. All studies consistently found a strong association between young maternal age and fetal gastroschisis.
The disproportionate number of young women whose pregnancies are complicated by gastroschisis and the overall increase in incidence worldwide suggest a role for underlying environmental causes, such as infection, nutrition, or medication use. Researchers have linked smoking, alcohol use, and use of common medications such as acetaminophen, aspirin, ibuprofen, and pseudoephedrine to gastroschisis. However, it is unclear if these associations are causal or sufficient to explain the increasing rates seen worldwide ( Table 20.1 ). Researchers have also found a significant association between mothers who reported both urinary tract infection and sexually transmitted infection in the month before conception or during the first trimester and fetal gastroschisis (adjusted odds ratio 4.0, 95% confidence interval 1.4–11.6). A more recent study evaluating smoking as a risk factor for gastroschisis found first-trimester cigarette exposure was more common in mothers with infants diagnosed with gastroschisis. However, after controlling for confounding variables (maternal age, preconception body mass index), the association between smoking and development of gastroschisis was determined to be weak, with an adjusted odds ratio of 1.6 (95% confidence interval 1.1–2.3).
| Association | Weight of Evidence |
|---|---|
| TERATOGENIC | |
| Tobacco | Weak |
| Alcohol | Weak |
| Cocaine | Very weak |
| Aspirin | Weak |
| Ibuprofen | Weak |
| Pseudoephedrine | Weak |
| Acetaminophen | Weak |
| EPIDEMIOLOGIC | |
| Young maternal age | Strong |
| Low maternal body mass | Weak |
| Short interval between menarche and first pregnancy | Weak |
| Low socioeconomic status | Weak |
| Poor maternal education | Weak |
| INFECTIONS | |
| Genitourinary infections | Weak |
Gastroschisis is rarely associated with aneuploidy and uncommonly linked to additional anomalies. There are isolated reports of gastroschisis in association with trisomies 13, 18, and 21 and monosomy 22. In the European surveillance of congenital anomalies (EUROCAT) registry of live births, stillbirths after 20 weeks’ gestation, and terminations, 3% of cases of gastroschisis had a chromosomal anomaly; however, the definition of gastroschisis included various abdominal wall defects. In a population-based study, after excluding other abdominal wall defects, 14% of cases of gastroschisis were associated with additional defects (central nervous system being the most common at 4.5%), and 1.2% of cases of gastroschisis were associated with abnormal chromosomes. Traditionally, gastroschisis is regarded as a nonfamilial anomaly; however, authors have reported familial recurrences of gastroschisis. In a population-based study in California that included an extended pedigree of all probands, six (4.7%) of 127 families had more than one affected relative, and the sibling recurrence rate was 3.5%. The incidence of gastroschisis in offspring of affected individuals is unknown, and the role of genetic, epigenetic, and environmental factors on the development of gastroschisis is also undetermined.
Etiology and Pathophysiology
Gastroschisis has been regarded as a disruption (i.e., an abnormality produced after initial normal development) rather than a malformation (i.e., an abnormality occurring during early embryonic development). However, emerging evidence suggests that gastroschisis may be a malformation. Several hypotheses have been proposed to describe events leading to the development of gastroschisis. All hypotheses involve defective formation or disruption of the body wall, leading to subsequent herniation of bowel ( Table 20.2 ).
| Year | Author | Hypothesis |
|---|---|---|
| 1963 | Duhamel | Failure of embryonic mesenchyme to form the abdominal wall because of teratogen exposure |
| 1975 | Shaw | Rupture of amnion around the umbilical ring during the time of physiologic herniation or later |
| 1980 | deVries | Abnormal involution of right umbilical vein leading to weakening of the body wall and subsequent gut herniation |
| 1981 | Hoyme et al. | Disruption of right omphalomesenteric (vitelline or yolk sac) artery leading to infarction and necrosis at the base of the cord with subsequent body wall damage |
| 2007 | Feldkamp et al. | Abnormal folding of body wall resulting in a ventral body wall malformation leading to herniation of fetal gut |
| 2009 | Stevenson et al. | Failure of yolk sac and related vitelline structures to become incorporated into the body stalk, orphaning the vitelline duct and yolk sac outside both the main body stalk and the abdominal wall |
Because a disproportionate number of fetuses with gastroschisis are carried by younger women, research has focused on factors thought to be more prevalent in this group, such as nutritional patterns and drug use. The generally accepted vascular pathogenesis of gastroschisis has prompted a focus on vasoactive factors such as cocaine use, smoking, and use of cold remedies. Study results have been largely contradictory, with weak or modest associations found between various environmental factors. More recently, other new theories have been presented to explain the mechanism behind the defect.
The first proposes that gastroschisis is the consequence of failure of one or more of the folds responsible for abdominal wall closure. This failure is the proposed mechanism for other ventral wall defects (e.g., ectopia cordis, cloacal exstrophy). The proposed mechanism states that the body fold failure impedes the merging of the yolk sac with the body stalk. As development of the gut continues, part of the primary intestinal loop attached to the vitelline duct herniates through the body fold defect and into the amniotic cavity instead of the umbilical cord. An alternative scenario is proposed in which the primary intestinal loop herniates normally into the umbilical cord, with another part of the gut herniating through the unclosed portion of the ventral wall.
Another proposed mechanism explaining development of gastroschisis was presented in an article by Stephenson et al. in 2009. These authors hypothesized that the determining defect in gastroschisis is failure of the yolk sac and related vitelline structures to be incorporated into the umbilical stalk. This failure leads to persistence of the vitelline duct and yolk sac outside the main body stalk and abdominal wall, whereas the lateral abdominal walls close normally. The developing midgut has two points of egress from the abdominal cavity; this leads to abnormal herniation of the expanding midgut into the amniotic cavity and subsequent development of gastroschisis.
Manifestations of Disease
Clinical Presentation
Nearly all cases of gastroschisis are associated with elevated levels of maternal serum alpha-fetoprotein (MSAFP). Before routine US screening, MSAFP may have been the sole prenatal indicator of gastroschisis. In a series of 23 cases of gastroschisis, the average MSAFP was 9.42 multiples of the median; MSAFP was elevated in every case.
Most cases of gastroschisis are now diagnosed prenatally with US. The EUROCAT study reported a 90% detection rate of gastroschisis on prenatal US. A subsequent population-based study detected 97.7% of cases during prenatal US. There are reports of diagnosis of gastroschisis during the late first trimester. However, most cases are diagnosed during routine anatomy US performed at 18 to 20 weeks’ gestation.
Imaging Technique and Findings
Ultrasound.
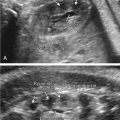
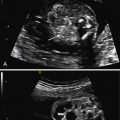
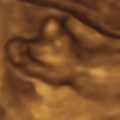
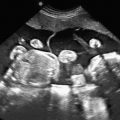
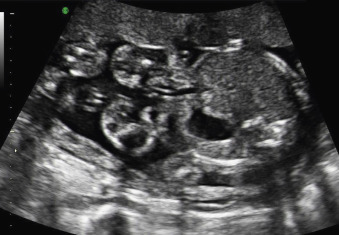
The classic appearance of fetal gastroschisis on US is free-floating loops of bowel within the amniotic fluid ( Fig. 20.3 ). On closer examination, a defect is seen to the right (in most cases) of a normally inserted umbilical cord ( ). The intestine is usually the only herniated organ. It is rare to see concomitant herniation of additional abdominal organs in cases of isolated gastroschisis. There is no peritoneal covering of the intestinal mass, and the exteriorized mass has a “cauliflower” appearance because the fluid between loops of bowel results in acoustic interfaces at both near and far bowel walls ( Fig. 20.4 ). Visualization of free-floating bowel loops is enhanced secondary to echogenic bowel wall edema and inflammation in addition to the dilated intestinal lumen ( Fig. 20.5 ). The stomach and intestine may become dilated because of obstruction (volvulus, atresia or stenosis, or malrotation) or hypoperistalsis.