Genital Tract and Bladder Ultrasound
William E. Brant
Female Genital Tract
US is the primary imaging modality for the evaluation of the female genital tract and pelvis (1). Indications for pelvic US examination include infertility, pelvic pain, disorders of menstruation, abnormal or limited physical examination, suspicion of mass or infection, and localization of intrauterine contraceptive device (IUD) (2). US is used as an adjunct to physical examination to confirm the presence or absence of a pelvic mass; evaluate its size, contour, and character; determine the organ of origin; evaluate for involvement of other organs; and detect the presence of ascites, hydronephrosis, and metastases. The US examination is usually initiated with a transabdominal approach using the distended urinary bladder as a window to the pelvis. The transvaginal US is performed with the bladder empty and provides the most detailed evaluation. Color flow US is used to identify pelvic blood vessels, identify vascular lesions of the pelvis, and to demonstrate tumor vascularity. Saline infusion sonohysterography (SHG) utilizes real-time US imaging of the uterus during injection of the uterine cavity with sterile saline to detect and characterize abnormalities of the uterus and endometrium (3).
Uterus
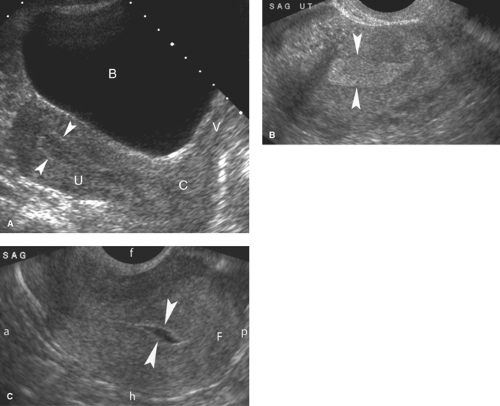
Normal US Anatomy. The uterus in the postpubertal woman is smoothly contoured and pear shaped (Fig. 36.1). The myometrium is uniform midlevel echogenicity, with the endometrium distinctly more echogenic. The thickness of the endometrial echo varies with menstrual state (4). The innermost myometrium, called the junctional zone, may appear as a thin hypoechoic layer adjacent to the echogenic endometrial stripe. Maximum normal uterine dimensions in the adult woman are 9 cm in length, 6 cm in width, and 4 cm in anteroposterior diameter. Following menopause, the uterus atrophies to approximately 6 × 2 × 2 cm. The prepubertal, infantile, uterus is cigar shaped. The cervix makes up about one-third the length of the uterus in the adult woman and about two-thirds of the length of the uterus in the prepubertal girl. Normal uterine positions in the pelvis include tilted forward (anteverted—most common), tilted backward toward the sacrum (retroverted), or folded anteriorly (anteflexed) or posteriorly (retroflexed) (Fig. 36.1C). The normal uterus may also be tilted right or left toward the pelvic sidewalls. The position of the uterus is altered by the degree of bladder filling and the presence of pelvic masses. The retroverted or retroflexed uterus appears more globular on transabdominal scanning. The normal vagina appears as a flattened muscular tube with echogenic mucosa.
The US examination must always be correlated with the state of the menstrual cycle, which affects the normal brightness and thickness of the endometrium (Fig. 36.1). At the end of menstruation, the endometrium is discrete and thin (2 to 3 mm). During the proliferative phase, the endometrium assumes a three-line appearance 4 to 8 mm thick. The basal endometrium, adjacent to the junctional zone myometrium, remains echogenic, whereas the functional endometrium, which will thicken and eventually slough with menstruation, is relatively hypoechoic during the first half of the menstrual cycle. The three lines are formed by the anterior and posterior basal endometrium and the echogenic stripe that marks the uterine cavity. Measurement of endometrial thickness is defined by the added thickness of the anterior and posterior endometrium. Any fluid or blood within the uterine cavity is excluded. At midcycle, the endometrium normally measures 8 to 10 mm in double-layer thickness. From ovulation to menstruation through the secretory phase, the endometrium progressively thickens up to 14 mm and becomes more uniformly echogenic. The junction zone myometrium appears as a hypoechoic halo surrounding the bright endometrium. In the normal postmenopausal woman, the echogenic endometrium does not exceed 5 to 7 mm in thickness. During the normal fertile years, pregnancy must always be considered in evaluation of the female genital tract. Abnormalities of the first trimester of pregnancy are reviewed in Chapter 37.
Arcuate artery calcifications are seen as discrete echogenic foci in the outer third of the myometrium of postmenopausal women. They are seen more commonly in women who are diabetic or hypertensive.
Congenital anomalies of the uterus result from impaired development, fusion, or resorption of the paired Müllerian ducts that evolve into the structures of the female reproductive tract. Müllerian duct anomalies are frequently associated with infertility. MR provides the most comprehensive imaging characterization. Uterine anomalies are reviewed more extensively in Chapter 34. US may define two uterine horns, two distinct endometrial cavities, and an abnormal shape of the uterus. The kidneys should be examined for associated anomalies such as renal agenesis.
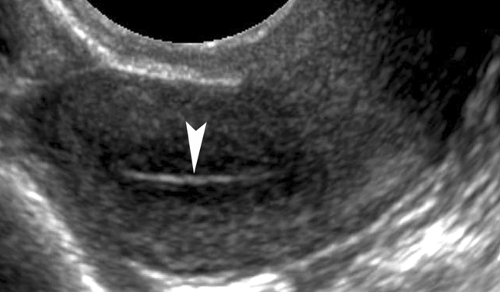
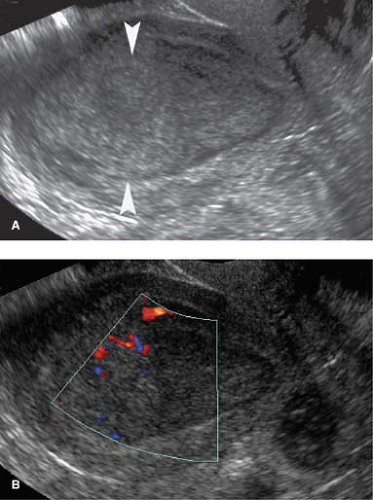
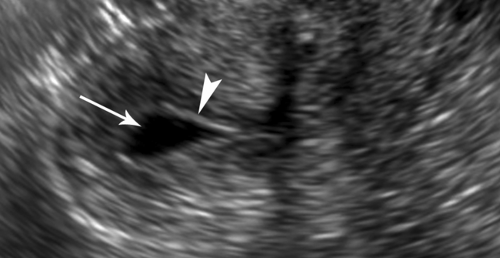
Leiomyomas (fibroids) are exceedingly common benign smooth muscle tumors of the myometrium that develop in women of all ages. They are suspected when the uterus is enlarged or altered in contour. Leiomyomas are virtually always multiple. They may be completely within the myometrium, subserosal or submucosal in location. Leiomyomas may also be pedunculated and predominantly extrauterine, simulating an adnexal mass. Color flow US demonstration of vascular supply contiguous with the myometrium (the bridging sign) is definitive in confirming uterine origin of these exophytic leiomyomas (5). Uncomplicated leiomyomas may be isoechoic, hypoechoic, or hyperechoic compared to normal myometrium (Fig. 36.2). A characteristic finding is “Venetian blind” shadowing, a pattern of spaced dark linear echoes (shadows) emanating from the leiomyoma, caused by increased absorption of sound by fibrous tissue within the tumor. This finding may be particularly useful in the differentiation of submucous leiomyomas from endometrial polyps. Atypical appearance of leiomyomas may result from atrophy, internal hemorrhage, cystic degeneration, fibrosis, and calcification (6). A “popcorn” pattern of calcification is characteristic and definitive on plain film radiographs. Lipoleiomyomas contain fat in addition to smooth muscle and connective tissue resulting in highly echogenic uterine masses. Retroposition of the uterus and uterine anomalies, such as a bicornuate uterus, must be differentiated from leiomyoma. Leiomyomas may cause menorrhagia or vaginal bleeding unrelated to menstrual cycles. Exophytic tumors may torse and may be a cause of acute pelvic pain. The tumors are responsive to female hormones and commonly accelerate in growth during pregnancy. Correspondingly, they involute with menopause. Symptomatic leiomyomas are treated with gonadotropin-releasing hormone analogs, selective uterine artery embolization, or focused US ablation, all
of which may result in tumor necrosis, internal hemorrhage, and cystic changes (7). No imaging modality can reliably differentiate the very common benign leiomyoma from the quite rare leiomyosarcoma.
of which may result in tumor necrosis, internal hemorrhage, and cystic changes (7). No imaging modality can reliably differentiate the very common benign leiomyoma from the quite rare leiomyosarcoma.
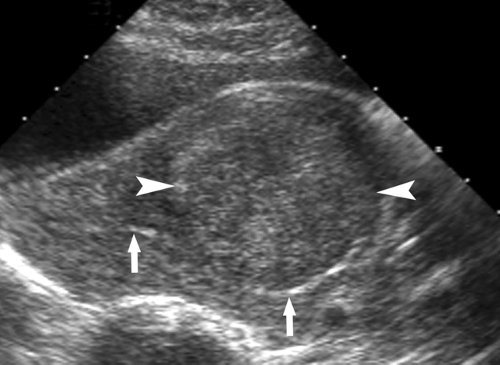 Figure 36.2. Leiomyoma. Transvaginal image of the uterus shows a hypoechoic leiomyoma (between arrowheads) displacing the endometrium (arrows) and impinging on the uterine cavity. |
Leiomyosarcoma is a malignant tumor composed entirely of smooth muscle. It is a primary sarcoma of the uterus. It is a rare tumor difficult to diagnose clinically. A rapid increase in the size of a uterine lesion in a postmenopausal woman is the most diagnostic clinical feature. Imaging features overlap with benign leiomyomas (Fig. 36.3). Diagnosis is made histologically.
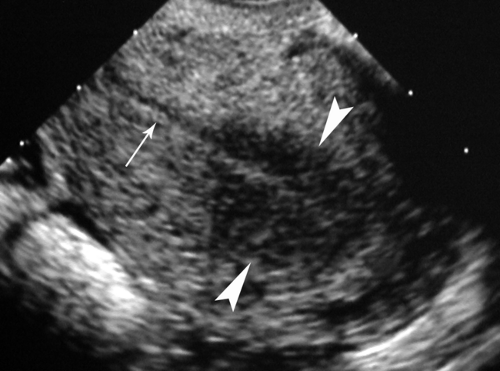
Adenomyosis is the condition of diffuse or focal invasion of the myometrium by benign endometrium (“internal endometriosis”). It is found commonly in multiparous women over age 30. The diffuse form is most common, with islands of endometrium scattered throughout the myometrium. The localized form results in a mass, an adenomyoma, within the myometrium. A broad spectrum of sonographic appearance is related to the distribution of ectopic endometrium, the presence and number of cysts within the ectopic endometrium, and the amount of associated myometrial hypertrophy. The most common US findings are (8) (1) diffuse abnormal hypoechoic or heterogeneous echotexture of the myometrium (Fig. 36.4), (2) poor definition or nodularity of the junction between endometrium and myometrium, (3) subendometrial echogenic nodules, (4) subendometrial myometrial cysts (1 to 5 mm), and (5) subendometrial hypoechoic linear striations. The uterus is usually enlarged. Leiomyomas are commonly present as well, frequently masking the coexistence of adenomyosis. MR provides the best detection and characterization of adenomyosis. See Chapter 34.
Thickened Endometrium. The thickness of the endometrium must always be correlated with age, menstrual history, and stage of the menstrual cycle. The full thickness of the echogenic endometrium, including both anterior and posterior endometrium, is measured perpendicular to the long axis of the uterus. In women having active menstrual cycles, the endometrial stripe may measure up to 14 to 16 mm during the secretory phase (4). However, in postmenopausal women, the endometrium normally does not exceed 5 mm in thickness.
Postmenopausal bleeding (PMB) occurs in 10% of postmenopausal women. The presence of endometrial cancer in 10% of women with PMB demands accurate evaluation (9). The most common cause of PMB is endometrial atrophy (70%) associated with a thin endometrium. Other common causes of PMB are associated with a thickened endometrium (Table 36.1). Transvaginal US is routinely used to assess the appearance and to measure the thickness of the endometrium (9). An endometrium thickness of 5 mm is generally accepted as a cut-off value. In the presence of PMB, the endometrium should be biopsied if double-layer thickness exceeds 5 mm. The risk of cancer is minute if the endometrial thickness is less than 5 mm. SHG is used commonly to further characterize lesions shown on US and to assess whether they are amenable to hysteroscopic resection (10).
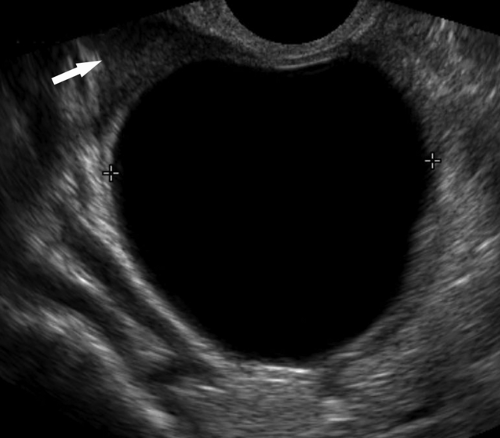
Endometrial atrophy is characterized by a uniformly thin endometrium with double-layer thickness less than 5 mm (Fig. 36.5). Thin endometrium is a normal and expected finding in postmenopausal women. However, in some women, the thin endometrium leads to superficial erosion and bleeding occurs.
The causes of thickening of the endometrium include the following:
Endometrial carcinoma may appear as diffuse thickening of the endometrium or as a focal endometrial mass. Endometrial thickness greater than 15 mm is strongly associated with carcinoma (Fig. 36.6). The endometrium is commonly heterogeneous, has uneven thickness, and an
ill-defined interface with the adjacent myometrium. PMB is the most common presenting symptom.
Table 36.1 Causes of Postmenopausal Bleeding
Common
Endometrial atrophy (70%)
Endometrial polyps (2%–12%)
Endometrial hyperplasia (5%–10%)
Endometrial carcinoma (10%)
Submucosal leiomyoma
Uncommon
Cervical carcinoma
Cervical polyps
Cervicitis
Tamoxifen therapy
Vaginal mucosal atrophy
“Rogue” ovulations
Endometrial hyperplasia is caused by unopposed or prolonged estrogen stimulation and is most common in perimenopausal and postmenopausal women (11). The endo-metrium is thickened and inhomogeneous, with small cysts commonly present. Only biopsy can differentiate endometrial hyperplasia from endometrial cancer.
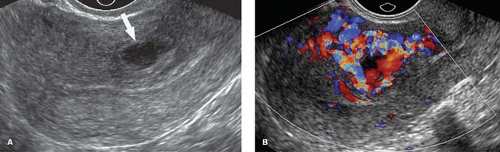
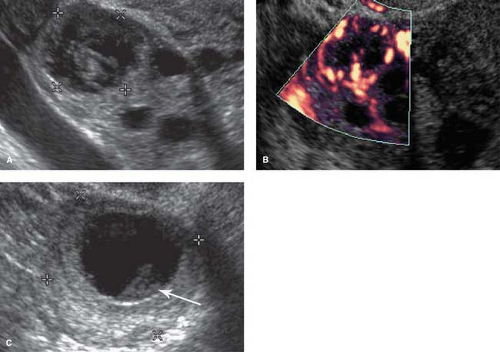
Endometrial polyps result from focal hyperplasia or adenomatous neoplasia of the endometrium. They are most common between ages 30 and 60. Malignant transformation is reported in 1% to 4%. About 20% are multiple. US demonstrates a focal echogenic polypoid mass in the endometrium (Fig. 36.7) or diffuse endometrial thickening. Compared to submucosal leiomyomas, endometrial polyps are homogeneously echogenic, smaller (<20 mm), and have a single feeding vessel (12).
Tamoxifen, used as an adjunct therapy for breast cancer, increases the risk of endometrial carcinoma two- to sevenfold (13). It is also associated with an increased incidence of endometrial polyps, endometrial hyperplasia, and sometimes striking cystic changes in the endometrium (Fig. 36.8).
Submucosal leiomyomas cause abnormal bleeding by erosion of the overlying endometrium. The most common
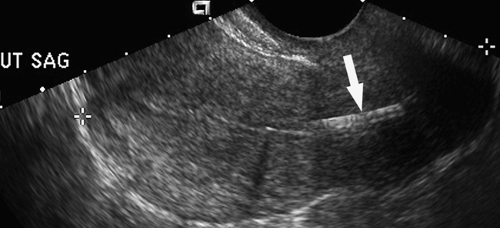
symptom is bleeding throughout the menstrual cycle. Compared to endometrial polyps, submucosal leiomyomas tend to be more hypoechoic, larger (>20 mm), and have multiple feeding vessels (12). Acoustic shadows emanating from the mass favor leiomyoma (Fig. 36.9). Lesions that protrude more than 50% of their diameter into the uterine cavity can generally be removed hysteroscopically.
The precise diagnosis is determined by endometrial biopsy. Pregnancy must never be forgotten as a possibility.
Fluid in the endometrial cavity may be blood, mucus, or purulent material. Hematometra refers to blood in the endometrial cavity and hematocolpos describes blood filling the vagina. In postmenopausal women, causes of fluid in the uterine cavity include cervical stenosis (Fig. 36.10), cervical carcinoma, endometrial carcinoma, endometrial polyps, and pyometrium. In premenopausal women, causes include congenital obstruction due to imperforate hymen; vaginal septum; vaginal or cervical atresia; acquired cervical obstruction due to instrumentation, radiation, or carcinoma; menorrhagia; and pregnancy.
Nabothian cysts result from the obstruction of the ducts of mucous-secreting glands of the epithelial lining of cervix and are commonly visualized on transvaginal US. They are usually anechoic, frequently multiple (Fig. 36.11), and vary in size (2 to 3 mm up to 4 cm). They are nearly always asymptomatic.
Uterine arteriovenous malformations (AVMs) are composed of a tangle of vessels of various sizes consisting of both arteries and veins but without an intervening capillary network (14). Congenital AVMs have a central nidus with multiple feeding arteries, large branches external to the uterus, and draining veins. AVMs acquired as a result of trauma, surgical procedures, gestational trophoblastic disease, or endometrial or cervical cancer tend to have single or few intrauterine artery feeders and lack the central nidus. Some patients are asymptomatic, whereas others have intermittent, sometimes torrential, bleeding. US shows a heterogeneous uterus with tubular anechoic spaces in the myometrium (Fig. 36.12). CDU shows a bright color mosaic of the vascular tangle. Spectral Doppler arterial waveforms are of high-velocity and low-resistance characteristic of arteriovenous shunting. Angiographic embolization is the treatment of choice.
Intrauterine contraceptive devices (IUDs) currently used in the United States are the T-shaped copper-wrapped ParaGard® IUD and the hormone impregnated T-shaped Mirena® IUD. Complications include expulsion, malposition, uterine perforation, infection, and pregnancy (15). Copper-wrapped IUDs produce prominent acoustic shadowing and reverberation echoes that make identification and localization easy. The Mirena is more difficult to identify, requiring careful US examination especially if the uterus is distorted by the presence of leiomyomas. The normal position of the IUD is centered within the uterine canal with the T-shape portion abutting the fundus. If the IUD is low in position in the mid or lower uterus, it is ineffective as a contraceptive (Fig. 36.13). Malposition with penetration of the myometrium or even perforation of the uterus is associated with pelvic pain. Expulsion of the IUD may not be noticed by the patient, except for absence of the IUD string, and is confirmed with US. If a pregnancy occurs, it is more likely to be ectopic. Infection (pelvic inflammatory disease [PID]) may complicate the use of IUDs.
Ovaries and Adnexa
Normal US Anatomy. The term adnexa refers to the ovaries, fallopian tubes, broad ligament, and ovarian and uterine
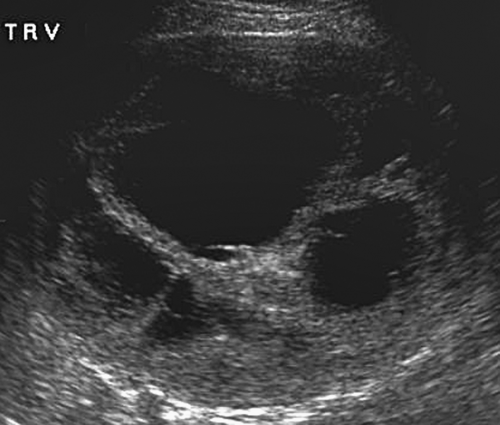
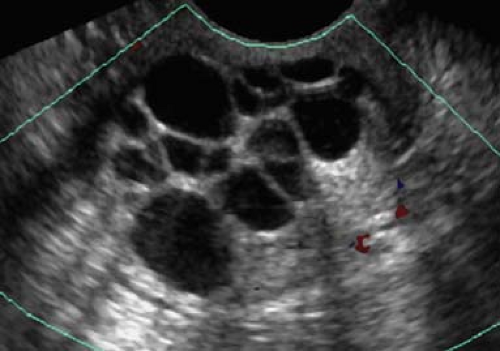
vessels, all of which may be involved in pathological conditions. US demonstrates the ovaries as oval soft tissue structures with multiple small cystic follicles. The ovaries average 4 × 3 × 2 cm in size, with a maximum of 5 cm in any one dimension. The maximum ovarian volume for an adult woman, calculated by the standard formula (length × width × height × 0.52), is 22 cc. The ovaries show characteristic morphological changes with the menstrual cycle. Following menstruation, the ovaries are at their smallest, with the follicles measuring less than 5 mm. During the estrogen phase, follicles enlarge to 10 to 15 mm size, with one dominant follicle attaining 20 to 30 mm size by midcycle (Fig. 36.14). Rupture of the dominant follicle releases the ovum and the corpus luteum forms at the site of the dominant follicle. Ovulation releases fluid which pools in the cul-de-sac. All remaining follicles normally involute following ovulation. Hemorrhage into the corpus luteum or any follicle produces a hemorrhagic functional cyst. The ovaries vary widely in location, but usually lie in a shallow ovarian fossa in the angle between the external iliac vessels anteriorly and the ureter posteriorly, with the fallopian tubes draped over and around them. The fallopian tubes are not visualized unless enlarged; however the broad ligament is clearly seen when it is outlined by fluid in the pelvis. Postmenopausal ovaries are atrophic, lack follicles, and are often difficult to visualize. Mean ovarian volume decreases from 8 cc at age 40 to 44 years to less than 1.0 cc at age 70 years. Maximum ovarian volume in a postmenopausal woman is 6 cc. In infants up to 24 months of age, the ovaries are small with a mean volume of 1 cc and a maximum volume of 3 cc. Focal calcifications in otherwise normal appearing ovaries are a common and benign finding.
vessels, all of which may be involved in pathological conditions. US demonstrates the ovaries as oval soft tissue structures with multiple small cystic follicles. The ovaries average 4 × 3 × 2 cm in size, with a maximum of 5 cm in any one dimension. The maximum ovarian volume for an adult woman, calculated by the standard formula (length × width × height × 0.52), is 22 cc. The ovaries show characteristic morphological changes with the menstrual cycle. Following menstruation, the ovaries are at their smallest, with the follicles measuring less than 5 mm. During the estrogen phase, follicles enlarge to 10 to 15 mm size, with one dominant follicle attaining 20 to 30 mm size by midcycle (Fig. 36.14). Rupture of the dominant follicle releases the ovum and the corpus luteum forms at the site of the dominant follicle. Ovulation releases fluid which pools in the cul-de-sac. All remaining follicles normally involute following ovulation. Hemorrhage into the corpus luteum or any follicle produces a hemorrhagic functional cyst. The ovaries vary widely in location, but usually lie in a shallow ovarian fossa in the angle between the external iliac vessels anteriorly and the ureter posteriorly, with the fallopian tubes draped over and around them. The fallopian tubes are not visualized unless enlarged; however the broad ligament is clearly seen when it is outlined by fluid in the pelvis. Postmenopausal ovaries are atrophic, lack follicles, and are often difficult to visualize. Mean ovarian volume decreases from 8 cc at age 40 to 44 years to less than 1.0 cc at age 70 years. Maximum ovarian volume in a postmenopausal woman is 6 cc. In infants up to 24 months of age, the ovaries are small with a mean volume of 1 cc and a maximum volume of 3 cc. Focal calcifications in otherwise normal appearing ovaries are a common and benign finding.
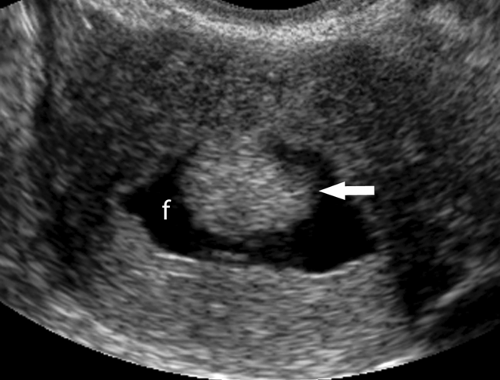
Follicles are normal physiologic structures on the ovary. Follicles are thin walled, contain anechoic fluid, and are arranged around the periphery of the ovary (Fig. 36.14). Normal follicles range up to 15 mm size, whereas the dominant follicle may be 30 mm in diameter just prior to ovulation. Follicles should be called follicles and should not be called cysts because “cyst” implies a pathologic finding (16).
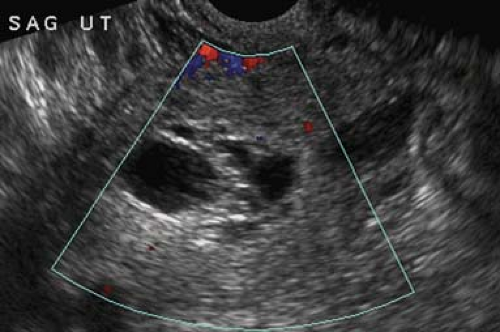
Normal corpus lutea are formed by rupture and collapse of the dominant follicle during ovulation. The corpus luteum functions secreting progesterone and estrogen. It initially appears as solid, vascular portion of the ovary (collapsed cyst appearance) (Fig. 36.15). It forms a small cystic mass (<3 cm) often with internal echoes, fluid levels, or meshlike internal structure (hemorrhagic cyst appearance). Its walls are typically thicker than the wall of normal follicles. Color Doppler shows an intensely vascular “ring of fire.” If pregnancy does not occur, the normal corpus luteum involutes. If it fails to involute, or if hemorrhage occurs, it enlarges to 4 to 5 cm to become a functional ovarian cyst or a hemorrhagic ovarian cyst. If pregnancy occurs, the corpus luteum persists as a physiologic cystic structure through 16 to 18 weeks gestation.
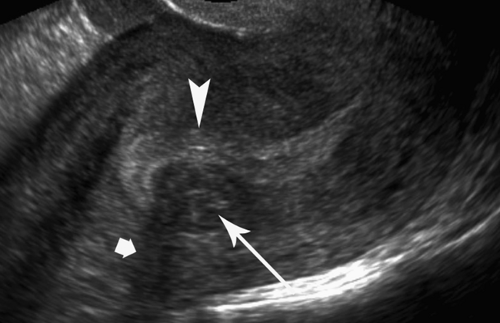
Functional ovarian cyst is the most common ovarian mass (Table 36.2). Small cysts, up to 3.0 cm, should generally be considered to be normal follicles (17). Pathologic follicular cysts up to 20 cm result from excessive accumulation of fluid or internal hemorrhage. They basically represent follicles or corpus lutea that fail to regress. Functional cysts may rupture or undergo torsion. Diagnosis is made by the demonstration of a round, smooth, usually unilocular ovarian cyst (Fig. 36.16) that resolves on follow-up examination after one or two menstrual cycles. Anechoic thin-walled cysts (simple cysts) that fail to resolve after two menstrual cycles may be neoplasms (cystadenomas or benign cystic teratomas); however they are extremely unlikely to be malignant. The Society of Radiologists in Ultrasound recommends yearly follow-up of “simple” adnexal cysts greater than 5 cm (17).
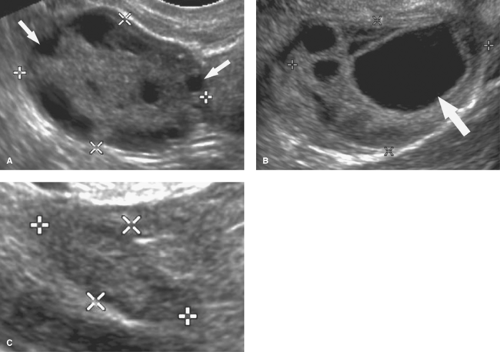
Hemorrhagic ovarian cysts result from hemorrhage into a follicle or the corpus luteum. Patients present with pelvic pain, often abrupt in onset, pelvic mass, or may be asymptomatic. Hemorrhagic ovarian cysts are common in premenopausal women and very rare in postmenopausal women unless they are taking hormone-replacement therapy. US shows a broad spectrum of findings (18): (1) the key finding is a cystic mass with internal echoes; (2) accentuated through-transmission reflects its cystic nature; (3) wall thickness is variable (2 to 20 mm); (4) blood flow in the wall is commonly prominent
and does not differentiate hemorrhagic cyst from tumor; (5) internal echogenicity depends upon the physical state of the hemorrhage; (6) the cyst may appear solid, but color flow US shows no internal blood vessels; (7) retracting clots adherent to the wall mimic neoplastic papillary projections but lack blood flow; (8) a web-like pattern of lacy internal echoes representing fibrin strands is characteristic (Fig. 36.17). Particulate matter within hemorrhagic cysts may demonstrate acoustic streaming described as the movement of particulate matter in fluid in the direction of the sound beam away from the transducer. Endometriomas, which may otherwise appear identical to hemorrhagic cysts, do not show acoustic streaming. Rupture of a hemorrhagic cyst causes acute pain and results in hemoperitoneum. Follow-up US usually shows complete resolution within two menstrual cycles.
and does not differentiate hemorrhagic cyst from tumor; (5) internal echogenicity depends upon the physical state of the hemorrhage; (6) the cyst may appear solid, but color flow US shows no internal blood vessels; (7) retracting clots adherent to the wall mimic neoplastic papillary projections but lack blood flow; (8) a web-like pattern of lacy internal echoes representing fibrin strands is characteristic (Fig. 36.17). Particulate matter within hemorrhagic cysts may demonstrate acoustic streaming described as the movement of particulate matter in fluid in the direction of the sound beam away from the transducer. Endometriomas, which may otherwise appear identical to hemorrhagic cysts, do not show acoustic streaming. Rupture of a hemorrhagic cyst causes acute pain and results in hemoperitoneum. Follow-up US usually shows complete resolution within two menstrual cycles.
Table 36.2 Causes of an Ovarian Mass | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Postmenopausal ovarian cysts are benign serous inclusion cysts found in 15% of asymptomatic postmenopausal women. US features are (1) small size less than 5 cm, (2) smooth thin walls of uniform thickness less than 3 mm, (3) anechoic fluid contents, and (4) absence of septations, nodules, or any soft tissue component. Over time, these cysts commonly change size or disappear. Cysts with these characteristics in postmenopausal women are extremely unlikely to be malignant. The Society of Radiologists in Ultrasound recommends yearly follow-up of postmenopausal cysts greater than 1 cm (17).
Pelvic inflammatory disease (PID) refers to acute or chronic inflammation of the fallopian tubes, ovaries, and pelvic peritoneum (19). Patients are usually in their teens and twenties and present with pain, fever, and vaginal discharge. Causes of PID include gonococcus, chlamydia, anaerobic bacteria, and tuberculosis. The disease runs a spectrum from endometritis to salpingitis to hydrosalpinx and tubo-ovarian abscess. In acute PID, US demonstrates a complex ill-defined adnexal mass that often includes a dilated, pus-filled fallopian tube, swollen ovary, and adhesions to adjacent structures (Fig. 36.18). Echogenic, purulent, fluid is usually present in the cul-de-sac. Chronic PID manifests as hydrosalpinx or peritoneal inclusion cyst.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree