Clinical Indications
Renal ultrasonography is a commonly employed imaging technique with many useful applications. These include, but are not limited to, evaluation of hydronephrosis, detection and surveillance of nephrolithiasis, characterization of focal renal lesions, and workup of renal failure and hematuria.1–3 While not without limitations, ultrasound offers many advantages over computed tomography (CT) and magnetic resonance imaging (MRI). Ultrasound does not involve ionizing radiation, which is significantly advantageous in certain populations such as pregnant patients and children. Ultrasound can be used safely in patients with allergies to iodinated contrast agents and in patients with cardiac pacemakers. Ultrasound does not carry a risk of contrast-induced nephropathy or nephrogenic systemic fibrosis, and is not limited by renal function. Imaging guidance by ultrasound is frequently employed in renal biopsies, nephrostomy tube, or ureteral stent placements, and intraoperatively in lesion resection and treatment.
Ultrasound is excellent in detection of hydronephrosis and useful in the workup of hematuria, particularly in low-and medium-risk patients as maintained by the European Society of Urogenital Radiology.4 In detection and surveillance of nephrolithiasis, ultrasound offers an alternative to CT imaging in more radiosensitive patients and those with body morphology conducive in sonographic imaging. Ultrasound provides a high level of accuracy in characterization of focal masses as solid, cystic, or mixed, and can be utilized in surveillance of focal renal lesions for stability in conjunction with CT and MRI. Doppler imaging can be applied in the evaluation of cystic or solid lesions and in other renal vascular applications such as identifying arteriovenous fistulas or malformations, screening for renal artery stenosis, detection of renal vein thrombosis, and calculation of resistive indices in the workup of obstructive and medical renal disease. The color comet tail artifact, or “twinkling” artifact, provides additional sensitivity to ultrasound in detection of calcifications, either related to stone disease or complex cystic lesions.
Despite its useful applications, ultrasound possesses many limitations as a modality in renal evaluation. In the setting of acute trauma, ultrasound may not be able to differentiate acute hemorrhage from urine and does not accurately stage the degree of renal injury.5,6 CT serves as the primary imaging modality of choice following blunt or penetrating trauma, and has the advantage of evaluating adjacent organs and vasculature for concurrent injuries. Ultrasound does not reliably visualize the ureter, thus limiting its evaluation of urolithiasis, an assessment more optimally performed by noncontrast CT imaging.7
 Normal Anatomy
Normal Anatomy
The kidneys lie in the retroperitoneum surrounded by Gerota fascia and perinephric fat. Each kidney generally weighs 150 g in the males and 135 g in the females. A typical kidney measures 10–12 cm longitudinally, 5 to 7 cm transversely, and 3 cm in the anteroposterior dimension. While the position of the kidney within the retroperitoneum varies by side, degree of inspiration, body position, and anatomic anomalies, the right kidney is usually located 1 to 2 cm lower than the left secondary to inferior displacement by the liver. Anatomically, a normal right kidney can be found in the space between the top of the first lumbar vertebra to the bottom of the third lumbar vertebra, while the left kidney is more superiorly located between the 12th thoracic vertebra and third lumbar vertebra.
The muscles surrounding the kidneys are similar on both sides. The lower two-thirds of the kidneys lie on the psoas muscles posteromedially. This results in the lower pole of the kidney lying anteriorly and laterally relative to the upper pole and rotating the medial aspect of the kidney by approximately 30 degrees. This anatomy is of particular importance while gaining ultrasound-guided percutaneous access to the kidney.
On the right side, the kidney is juxtaposed with a number of structures. The liver lies adjacent to the upper pole both superiorly and anteriorly. The adrenal gland is also encountered in the superomedial aspect of the right kidney. Medially, the duodenum is adjacent to the hilum and hilar structures. The anterior aspect of the lower pole of the kidney is also covered by the hepatic flexure of the colon. On the left side, the spleen and adrenal gland are located at the superior and superior-medial aspects of the upper pole, respectively. The tail of the pancreas and splenic vessels are adjacent medially to the upper pole and renal hilum. Anteriorly the left kidney is covered by the splenic flexure of the colon.
The renal vasculature normally consists of one artery and one vein entering the kidney at the hilum, although occasionally multiple arteries and veins do exist. The renal artery divides into five segmental arteries: an anterior branch, which further subdivides into the apical, upper, middle, and lower segmental arteries, and the posterior branch. Renal arteries are end arteries with no communication between the various branches. The venous structures generally accompany the arteries through the renal parenchyma; however, unlike the arterial system they communicate freely. The left renal vein receives drainage from the adrenal, gonadal, and posterior lumbar veins before crossing midline anterior to the aorta to communicate with the vena cava. No other veins consistently drain into the right renal vein, although occasionally the right gonadal vein may end in the right renal vein instead of its typical drainage into the vena cava.
The renal parenchyma comprises a renal cortex and medulla. The medulla is composed primarily of the collecting ducts, while the cortex houses the majority of the nephrons. Each nephron is associated with an afferent arteriole that enters the glomerulus for filtration to take place. The filtrate from the glomerulus is reabsorbed and concentrated as it passes through the renal tubular system before it finally enters the collecting duct. Multiple collecting ducts make up a pyramid, and the tip of a pyramid is called the papilla. There are generally 7 to 9 papillae within a kidney and each is located within a renal calyx. The minor calyces are connected by infundibulae to form two or three major calyces, which join into the renal pelvis.
The renal pelvis lies posterior to the renal vessels and drains into the ureter. The ureter is generally 22–23-cm long and courses just medial to the psoas muscle in the retroperitoneum. The ureters cross anterior to the common iliac vessels near their bifurcation. The ureters then travel posterior to the gonadal vessels and drain into the bladder.
 Normal Appearance on Ultrasound
Normal Appearance on Ultrasound
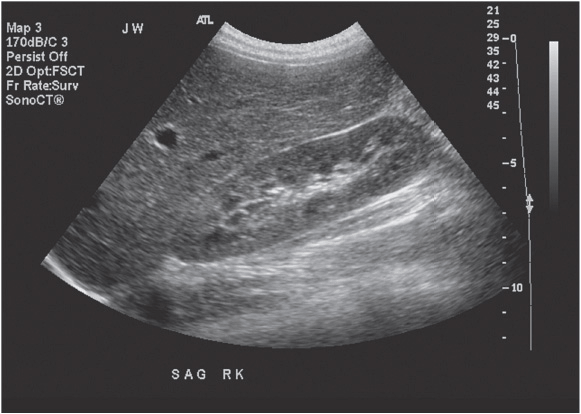
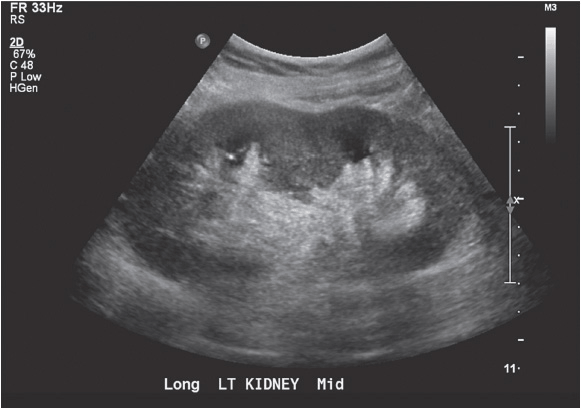
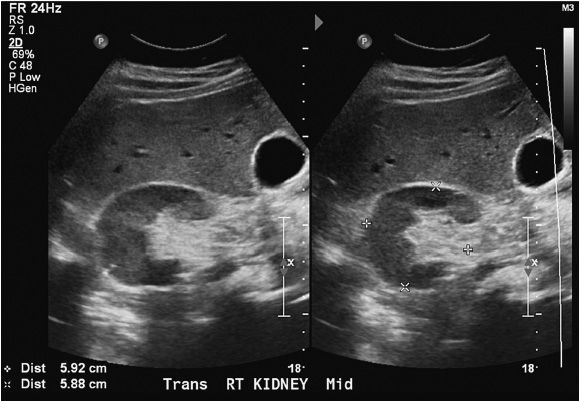
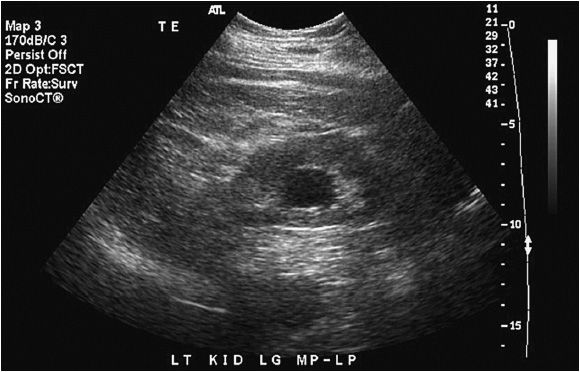
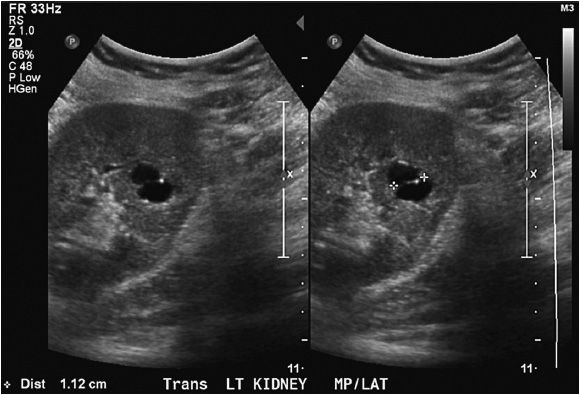
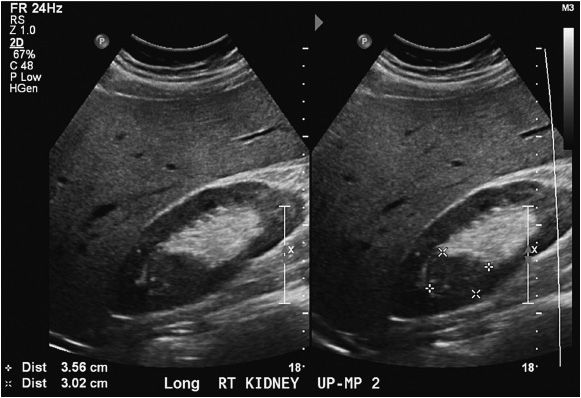
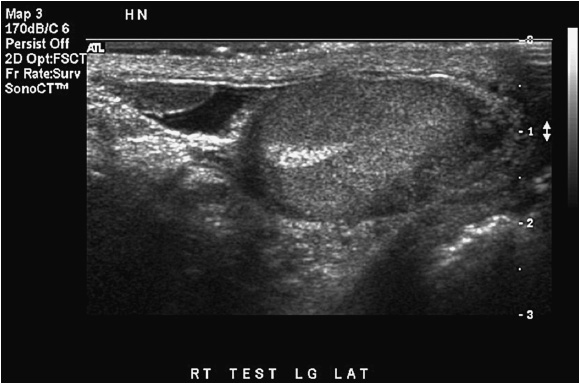
The normal kidney appears as elliptical on longitudinal views and rounded on transverse views with the renal parenchyma encasing the central renal sinus. The renal cortex is homogeneous and slightly hypoechoic compared to adjacent liver or spleen. The renal sinus is densely echo-genic due to its typical fat composition and contains the renal vessels, lymphatics, and renal pelvis. The renal medulla may occasionally be differentiated from the renal cortex as small triangular or rounded hypoechoic structures between the cortex and renal sinus fat. The renal capsule provides a strong acoustic interface with the surrounding perinephric fat providing a distinct demarcation of the renal outline and contour (Figures 13-1 to 13-3).
Figure 13-1. Longitudinal image of a normal right kidney. In this image, the liver provides a clear acoustic window for imaging the kidney. The renal sinus fat located in the center of the kidney is clearly distinguished from the renal parenchyma by its hyperechoic echotexture.
Figure 13-2. Longitudinal image of the left kidney. The gap in the renal outline is the renal hilum. That it is visible indicates that the probe is oriented appropriately to the position of the kidney.
Figure 13-3. Transverse images of the right kidney. Note the crescent shape of the normal kidney in the transverse view.
 Scanning Technique
Scanning Technique
A real-time 2–5-MHz frequency sector or curvilinear transducer is routinely utilized in renal imaging. Each kidney is thoroughly evaluated in both longitudinal and transverse planes. A maximum measurement of renal length should be documented for each kidney in addition to anteroposterior and transverse dimensions, when feasible, at the midpoint of the kidney. Images including adjacent liver and spleen are useful in assessing renal parenchymal echogenicity. We routinely begin imaging in the longitudinal plane before turning to the transverse plane. In keeping with standard ultrasound imaging convention, transducer orientation is maintained such that the left side of the screen displays more superior and right-sided structures in longitudinal and transverse planes, respectively.
The right kidney is typically easier to visualize in its entirety than the left, since the adjacent liver serves as an acoustic window, a feature not as well provided by the spleen in imaging the left kidney, making it often obscured or incompletely visualized. The examination on the right begins by placing the probe just anterior to the midaxillary line below the ribs and proceeding with longitudinal image acquisition, scanning the entire kidney from medial to lateral. The probe is subsequently rotated 90 degrees and transverse scanning is performed from the upper pole to the lower pole. Any abnormalities are documented and measured. The study is repeated for the left side but with the probe placed posterior to the midaxillary plane to take advantage of what acoustic window can be provided by the spleen and to minimize obscuration by gas at the descending colon and small bowel. Scanning and documentation proceed as with the right side. For imaging of the right kidney, the patient can be in supine or left lateral decubitus position. The left kidney is often better visualized with the patient in right lateral decubitus position to reduce obscuration by overlying bowel gas.
 Common Findings/Abnormalities
Common Findings/Abnormalities
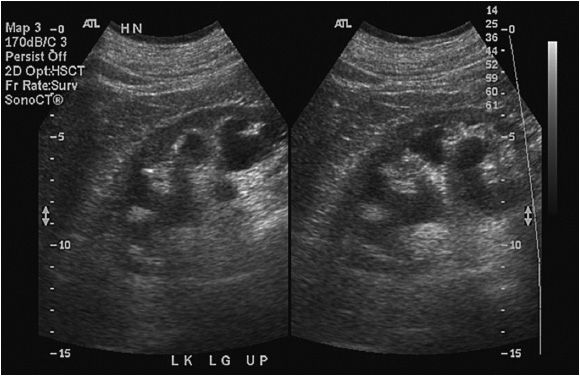
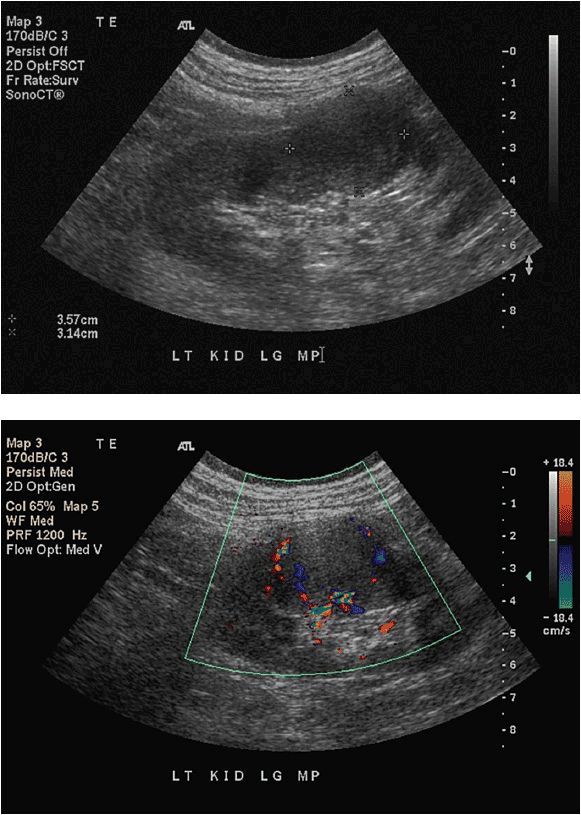
The most common abnormalities diagnosed by ultrasound include hydronephrosis and masses, both cystic and solid. Hydronephrosis can occur as a result of normal physiologic processes (eg, pregnancy) or obstruction from either an intrinsic process (eg, stone, stricture) or an extrinsic process (eg, retroperitoneal or pelvic malignancy). Ultrasound is ideally suited for diagnosing hydronephrosis with a sensitivity and specificity approaching 100%.8 In hydro-nephrosis, urine does not effectively drain from the renal pelvis, causing a sonolucent expansion in the central kidney superimposed on echogenic renal sinus fat. The sonolucent dilated renal pelvis can be distinguished from sonolucent hilar blood vessels by its lack of signal on Doppler imaging. As the hydronephrosis progresses, the renal parenchyma and sinus fat become compressed and the individual calyces become more evident. In severe hydronephrosis, there is parenchymal thinning and loss of the central echogenic sinus (Figure 13-4).9
Figure 13-4. Hydronephrosis. Prolonged hydronephrosis due to obstruction can eventually lead to parenchymal thinning as noted in this image.
Among the most common indications for renal ultrasound is the differentiation between solid and cystic masses of the kidney. Renal ultrasonography distinguishes solid from cystic masses with a diagnostic accuracy of over 98%.10 In order for a lesion to be diagnosed as a simple cyst, strict criteria must be met.8
1. Anechoic lesion
2. A well-defined back wall
3. Imperceptibly thin cyst wall
4. Increased through transmission with posterior acoustic enhancement
5. Uniform round or oval shape
If these criteria are not strictly met, then the lesion should be considered complex and further evaluation with CT or MRI is warranted (Figures 13-5 and 13-6).
Figure 13-5. Simple renal cyst. The cyst meets the criteria for a simple renal cyst in that it is a round, anechoic lesion with through transmission, an imperceptibly thin cyst wall, and a well-defined back wall.
Figure 13-6. Complex cyst. As opposed to simple cysts, complex cysts often have septations, calcifications of the wall and/or septae, wall thickening, and an abnormal contour.
While simple renal cysts of only a few millimeters in diameter can be easily distinguished from surrounding normal parenchyma, solid lesions can be better identified as they approach 2 cm in size.9,11 The detection rates for lesions 0–5 mm, 5–10 mm, 10–15 mm, 15–20 mm, 20–25 mm, and 25–30 mm are 0%, 21%, 28%, 58%, 79%, and 100%, respectively.11 The typical solid renal mass is hypoechoic to the surrounding normal parenchyma, but can be hyperechoic. While small renal masses are usually round or ovoid, in contrast to simple cysts, they do not have a well-defined back wall and do not display posterior enhancement due to increased through transmission (Figure 13-7).
Figure 13-7. Small renal mass. Small renal masses, as seen here, are characterized by their ovoid shape and solid appearance. There is no through transmission, a characteristic associated with cystic lesions.
On Doppler ultrasound, the mass can demonstrate either increased or decreased flow. Unfortunately, there are no characteristic findings that can distinguish the benign solid renal mass from a malignant renal mass (Figures 13-8 and 13-9). All solid renal masses identified by ultrasound are potentially malignant, and further investigation with either CT or MRI is recommended.
Figures 13-8 and 13-9. CFD of small renal mass. The addition of color flow Doppler cannot help distinguish malignant versus benign lesions, but can help further characterize the lesion. In this image, the mass (seen in Figure 13-8) demonstrates increased vascularity especially near the periphery of the lesion.
 Common Pitfalls
Common Pitfalls
As discussed above, application of Doppler will aid in distinguishing between dilated collecting system and renal vessels at the central kidney, both of which can appear sonolucent by gray scale evaluation, but only the vessels normally display signal on Doppler imaging. Strict adherence to features of a simple renal cyst is important in avoiding the common pitfall of misinterpreting a hypoechoic, potentially neoplastic, solid lesion as a cyst and the less critical error of misrepresenting hypoechoic medullary pyramids as cysts. In the setting of renal parenchymal disease where kidney echogenicity is increased obscuring the borders of the renal cortex from surrounding perinephric fat or in other cases of obscuration of the renal outline, it is important to recognize the limitations in ruling out contour deforming peripheral renal lesions as the kidney contour may not be readily defined. In such cases of less than optimal imaging where there is a high clinical importance in excluding the presence of a focal renal mass lesion, CT or MRI should be utilized.
 Clinical Pearls and Tips
Clinical Pearls and Tips
The twinkling artifact is a phenomenon encountered with color Doppler imaging whereby a strong specular reflector, such as a renal stone or other calcification, produces rapidly alternating blue and red color Doppler signals behind the object of interest. This finding mimics the presence of turbulent flow but is aptly denoted as an artifact as it does not reflect true flow but rather is produced as a result of ultrasound machine settings and surface roughness of the target lesion. The artifact is often valuable in detection of renal stones that do not produce a strong shadow due to their size and/or composition and also in the characterization of complexity in cysts that may contain internal or mural calcifications.12–15
SCROTAL ULTRASOUND
 Clinical Indications
Clinical Indications
Ultrasound is the ideal method for the evaluation of a wide variety of scrotal and testicular complaints including scrotal and testicular masses, hydroceles, spermatoceles, and varicoceles.16–20 Color flow Doppler (CFD) aids in evaluation of the acute scrotum, clinically defined as a painfully enlarged scrotum.21–26 While there are many causes for scrotal pain, the most ominous is testicular torsion. In torsion, the testicle twists on its own vascular pedicle effecting vascular congestion, pain, scrotal enlargement, and testicular ischemia. If not treated expediently, torsion results in testicular necrosis and eventual loss. CFD rapidly distinguishes testicular torsion from inflammatory causes of scrotal pain by identifying the characteristic loss of intratesticular blood flow. Other causes of the acute scrotum include epididymitis and orchitis, both of which can clinically mimic testicular torsion but are ultrasonographically distinguishable from torsion by a characteristic high blood flow, low resistance state on CFD.27 Ultimately, however, if testicular torsion is clinically suspected, immediate surgical intervention is mandatory regardless of ultrasound findings.
High-frequency scrotal ultrasound is also a key element in the evaluation of nonpainful scrotal enlargement. Among the various causes, the most ominous is the palpable testicular mass. Any patient complaint of a palpable testicular mass or a mass palpated on routine physical examination should be investigated. With a sensitivity for detecting intratesticular masses that approaches 100%, ultrasonography is the ideal initial imaging study that should be obtained.28, 29 Characteristics such as location, echogenicity, size, evidence of extratesticular extension or extratesticular location, and blood flow within the mass should be well documented. The ultrasonographic demonstration of an intratesticular mass requires surgical removal of the affected testicle due to the high probability of malignancy. Other causes of the nonpainful enlarged scrotum include hydroceles and spermatoceles, both of which may be managed expectantly or surgically, depending on the clinical scenario.
Scrotal ultrasonography is also indicated in the evaluation of varicoceles, especially in the pediatric population for assessment of testicular size. A difference in testicular volume of 20% on the affected side is an indication for varicocele ligation. Scrotal ultrasound has also been used in the diagnosis of traumatic testicular rupture. There are many causes of scrotal trauma with sports-related injuries accounting for approximately 50% and motor vehicle accidents 9–17%.30–33 Some studies have shown ultrasound to provide a sensitivity of 100% and specificity of 80% in the diagnosis of testicular rupture.34 As in the setting of torsion, however, when rupture is clinically suspected or ultrasound results equivocal, surgical exploration and repair is indicated.
 Normal Anatomy
Normal Anatomy
The scrotum comprises two laterally located chambers divided from each other by a midline scrotal septum.35,36 Within each chamber reside the testicle, epididymis, and components of the spermatic cord, namely the vas defer-ens and testicular arteries and veins. The testicle is made up of up to 400 lobules that converge at the mediastinum testis. The head of the epididymis (caput epididymis) arises from the superolateral aspect of the testicle and continues inferiorly to form the body of the epididymis (corpus epididymis) followed by the tail of the epididymis (cauda epididymis), located inferolaterally. The vas deferens arises from the tail and extends superiorly where it joins the testicular vasculature and passes into the inguinal canal. A thick fibrous capsule known as the tunica albuginea covers the testicular parenchyma. Two thin fascial layers (visceral and parietal tunica vaginalis) derived embryologically from the intra-abdominal peritoneum surround the tunica albuginea. These two layers normally produce and resorb a small (1–2 mL) amount of fluid. It is between these layers that a hydrocele can form as a consequence of excessive production or decreased resorption of fluid. This point marks the beginning of the scrotal wall, which surrounds both the testis and the cord structures. Extending to the skin in successive layers are the internal spermatic fascia, the cremasteric muscle, external spermatic fascia, dartos fascia, and skin.
The major blood supply to the testicle comes from the testicular arteries, which originate from the aorta. In the spermatic cord, the testicular arteries run alongside the cremasteric and deferential arteries, which provide blood flow to the cremasteric muscle and the vas defer-ens, respectively. The testicular artery branches into capsular arteries, which run just deep to the tunica albuginea.36 These capsular arteries branch further and penetrate into the testicle parenchyma, providing blood to the deeper structures. The venous drainage of the testicle coalesces at the mediastinum testis and, along with the venous drainage of the epididymis, forms the pampiniform plexus. The plexi drain into the right and left testicular veins that course through the abdomen in close relation to the testicular artery. The left testicular vein drains into the left renal vein, and the right testicular vein drains into the inferior vena cava.
 Normal Appearance on Ultrasound
Normal Appearance on Ultrasound
The scrotal wall generally appears slightly hypoechoic to the testicle and is typically 3–4 mm in thickness.8 The testicle itself is a homogenous ovoid structure approximately 5 cm in length, 3 cm wide, and 3 cm in anteroposterior diameter. The testicular parenchyma has a homogenous echotexture except at the mediastinum testis, where the rete testis can appear slightly hyperechoic (Figures 13-10 and 13-11).27 The epididymis is approximately 7 cm long in the adult male and can be seen on the posterolateral aspect of the testis. The head of the epididymis can sometimes appear slightly hyperechoic to the testis, while the body and tail of the epididymis can appear hypoechoic (Figure 13-12).27,37
Figure 13-10. Normal longitudinal ultrasound of the testis. The mediastinum testis is often clearly visualized due to the hyperechoic appearance of the rete testis. The normal testis is ovoid and homogeneous.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree