Gestational Trophoblastic Neoplasia
Includes
• Hydatidiform mole—Complete hydatidiform mole (CHM) and partial hydatidiform mole (PHM) (Table 24.1)
• Invasive mole (chorioadenoma destruens)
• Coexistent complete mole and live fetus
• Choriocarcinoma
• PSTT—Placental site trophoblastic tumor
Vaginal bleeding
Uterine enlargement more than expected for gestational age (GA)
Abnormally high hCG levels
Prevaginal passage of vesicles
Low levels of maternal serum alpha-fetoprotein (MSAFP)
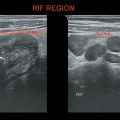
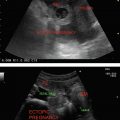
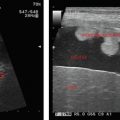
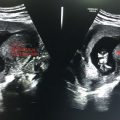
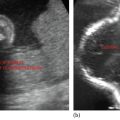
Enlarged uterine cavity filled with heterogeneous mass with cystic spaces of varying sizes (BUNCH OF GRAPES appearance) (Figure 24.1)
• No fetal development in complete mole.
• B/L theca—lutein cysts in enlarged ovaries.
•
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree