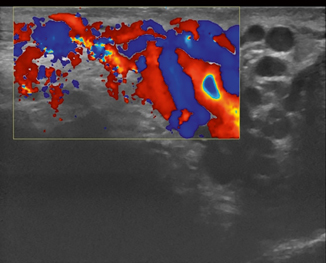
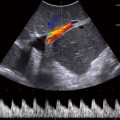
Fig. 16.1
Schematic illustration of the scrotal anatomy: 1 spermatic cord; 2 pampiniform plexus; 3 tunica vaginalis (outer, parietal layer); 4 epididymal appendix; 5 testicular appendix; 6 testicle covered by tunica albuginea; 7 testicular mediastinum; 8 seminiferous tubules; 9 epididymis (head); 10 ductus deferens; 11 testicular artery; 12 scrotal skin
The appendix testis is a remnant of the Müllerian duct, which is attached to the upper pole of the testis and the appendix epididymis is a remnant of the mesonephron, which is found on the epididymal head [1, 2].
Scanning Techniques
Position of the Patient
The patient is positioned in the supine position with the legs slightly spread apart. To improve exposure and to immobilize the testes, the scrotum is supported and slightly elevated by placing a towel beneath the scrotal sac. Ideally, it is placed in a towel sling. Prior to beginning with the ultrasound examination copious ultrasound jelly is applied to the scrotal area to provide sufficient surface interface of the linear transducer to the curvy scrotal surface and to avoid pressure on the testicle during the examination. It is very important for the child’s comfort that the applied ultrasound gel is warmed close to body temperature. Utilizing cold gel may cause contraction of the scrotal skin or may elucidate the cremaster reflex with subsequent ascent of the testicles. Both effects may compromise the ultrasound examination.
Scanning Techniques
The position of the scrotal and inguinal contents close to the surface requires use of a high-spatial resolution linear transducer dedicated to the study of soft tissues (9–15 MHz). Preferably, the transducer and the ultrasound software can provide simultaneous power and spectral Doppler ultrasonography as well.
The scrotum and its contents are scanned in different planes and documented in at least the long and short axes of the testicle (longitudinal and transverse scans). If one of the scrotal compartments is affected only, the unaffected hemiscrotum is scanned initially for comparison. The transducer is moved smoothly and slowly, examining all aspects of the anatomy.
Normal Sonographic Findings
The normal testis shows a granular medium-level echogenicity and is homogenous in appearance (Fig. 16.2). The testicular tissue echogenicity, in general, is similar to that of the liver or the normal thyroid gland [1, 2]. Prepubertal testes have a slightly decreased echogenicity relative to those in adults.
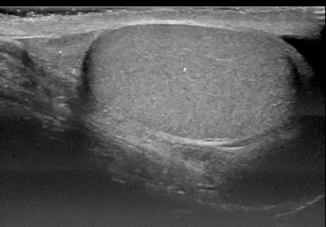

Fig. 16.2
Longitudinal scan of a pubertal testicle demonstrates granular homogenous echogenicity (# testes; * head of epididymis)
The tunica vaginalis appears as an echogenic outline of the testicle and a fold of it invaginates to the mediastinum of the testis. The mediastinum of the testis is visualized by ultrasound as a hyperechoic linear band running in craniocaudal direction.
The tubular rete testis appears as multiple small spherical or tubular hypoechoic regions close to the mediastinum testis.
The epididymis has an echogenicity slightly increased relative to the testicle. The epididymal head is round or ovoid in shape.
The appendix testis and the appendix epididymis are both embryological remnants of small ovoid hyperechoic protuberances found at the superior pole of the testis, normally hidden by the epididymal head. Unless torsed and swollen or outlined by fluid from a hydrocele , they are difficult to visualize on ultrasound.
In spectral Doppler sonography, the testis demonstrates a low-resistance arterial waveform. Color and spectral Doppler parameters should be set for low-flow power.
Some authors suggest that both, a short axis grayscale and a color Doppler image should be obtained of both testicles at the same time (“buddy shot” or “sunglasses view”), to compare relative echogenicity and blood flow. Most of the time, however, the transducer will not face both testicles at identical levels at the same time.
Size of the Testicle
The size of the testicle varies with age, increasing in size from birth to puberty. In a period of prepuberty, the testis contains a volume of about 2 cm3 while it may develop a volume of up to 12–14 cm3 during puberty [1].
In a population study of 769 boys (mean age = 9.0 years; range: 0.6–19.0 years), Goede et al. [3] found no significant volume differences between the left and right testicle. Boys who had been born prematurely or who had an ethnic background other than Caucasian showed testicular volumes comparable to boys who had been born at term or who had Caucasian ethnicity. The mean testicular volume at 1 year of age was 0.48 ml. At 10 years of age it was 0.97 ml in their cohort [3].
Others also found no significant volume differences between the left and right testicle, and between the different ethnical groups. There were also no significant effects found correlating with birthweight and current body weight [4].
Volume Measurement Equations
The methodology for assessing testicular volume is not standardized. In the literature, the volumes obtained by ultrasound have been calculated by different equations for the ellipsoid.
The commonly used formula to measure the volume of a prolate ellipsoid, such as the testicle [4], has been described as:
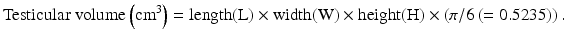
Further used equations are:
The Hansen formula, in which W is squared, where:
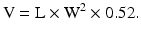
The Lambert formula [4], where:
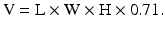
However, there is no agreement which formula is the most accurate and the use of these mathematical equations is currently debated. Authors from Taiwan [5] state that larger errors result when applying the Lambert equation to estimate the volume of smaller testes. The authors conducted an interesting study to evaluate the accuracy of different equations for assessing testicular volume of smaller testes. The equations used usually for the testicular volume calculations were compared with the gold standard volume measurement obtained by water displacement (Archimedes principle). In their results, testicular volume estimates obtained from ultrasound measurements (Hansen and Lambert equations) correlated better with those obtained by water displacement measurement.
The authors suggest in their conclusion a new constant (0.59) for use in the Hansen equation to assess the volumes of smaller testes most accurately (V = L × W2 × 0.59 [5]).
Undescended Testicle
Undescended testicles, located in the groin , or retractile testes are usually diagnosed by physical examination. There is no “true” indication for ultrasound examination routinely except for preoperative volume assessment and postoperative follow-up.
If the testicle is not found either in the scrotum or in the groin by physical examination (impalpable testicle; ectopic-, vanishing-, abdominal testes; testicular agenesis; true hermaphroditism), ultrasound is the first imaging modality performed. Twenty percent of the maldescended testes are non-palpable. Of the non-palpable testes, 50 % are abdominal, 45 % are atrophic, and 5 % are in the inguinal canal [6].
Imaging modalities including the MRI usually fail to detect non-palpable testis unless possibly located in the vicinity of the internal ring. The value of ultrasound for evaluation of non-palpable testes as discussed in the literature is controversial.
While Kanemoto et al. [7] and Wolverson et al. [8] showed that ultrasound had a sensitivity of 76–88 %, a specificity of 100 %, and an accuracy of 84–91 % for the diagnosis of non-palpable testis, others demonstrated limited diagnostic accuracy of imaging studies in non-palpable testes. Trasian et al. [9] conducted a meta-analysis including 12 studies of subjects younger than 18 years who had preoperative ultrasound evaluation for non-palpable testes and whose testes position was subsequently determined by surgery. They found that ultrasound had a sensitivity of 45 % and a specificity of 78 %, and concluded that ultrasound does not reliably localize non-palpable testes and does not rule out intra-abdominal testes.
Adesanya et al. [10] estimated the diagnostic accuracy of ultrasound evaluation for preoperative localization of undescended testes in children around 86.5 %.
Ultrasound is limited particularly in the evaluation of abdominal and retroperitoneal ectopic testes. For detection and evaluation of an atrophic testis, ultrasound may be inconclusive as it is difficult to differentiate the atrophic testicle from lymph nodes or parts of the infravaginal gubernaculum. Both structures can be mistaken for testicular tissue.
In our own experiences, diagnostic laparoscopy is the method of choice for suspected abdominal and non-palpable testes.
Shah and Shah [11] noted an overall diagnostic agreement between ultrasonography and laparoscopy in only 19 % of cases.
The undescended testicle might be smaller in volume and slightly hypoechoic in structure by ultrasound imaging compared to the normal testicle. The presence of a mediastinum testis (a longitudinal echogenic band) is diagnostic for the maldescended testis.
Hydrocele Testis, Spermatic Cord Hydrocele, Hydrocele of the Canal of Nuck
Hydrocele testis, spermatic cord hydrocele (funicolocele), and the hydrocele of the canal of Nuck (in girls only) are seen as anechogenic homogeneous fluid collections by ultrasound imaging (Fig. 16.3), occasionally containing septations and loculations (Fig. 16.4), calcifications and cholesterol.
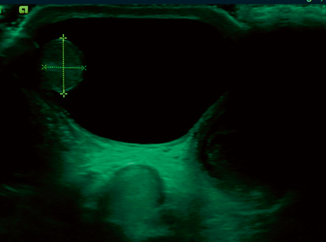
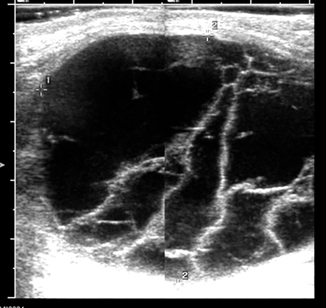

Fig. 16.3
Transverse sonogram shows the testis within a large, anechoic hydrocele (clear fluid collection)

Fig. 16.4
Ultrasound image of a very large hydrocele containing multiple septations and loculations
Nuck’s cyst presents as a cystic mass lying superficially and medially to the pubic bone at the level of the superficial inguinal ring enlarging toward the major labia. By clinical examination, Nuck’s cysts may mimic an ovarial prolapse in female infants and a funicolocele (hydrocele of the cord) may mimic an irreducible inguinal hernia in boys. In those cases, a quick ultrasound evaluation brings clarity to the diagnosis very easily and can avoid repeated attempts of unsuccessful reduction by a physician, who is unfamiliar or inexperienced with these conditions.
Acquired or reactive hydroceles are seen in conjunction with infection, tumors, trauma, torsion, and incarcerated inguinal hernia.
The abdominoscrotal or communicating hydrocele represents a rare condition in children where a large scrotal hydrocele is connected with the abdominal cavity. The abdominal extension of the fluid collection may be preperitoneal or retroperitoneal, and the processus vaginalis may be patent or obliterated. The disorder has been associated with a variety of pathological entities (Fig. 16.4).
Varicocele
A varicocele is defined as a collection of abnormal enlarged tortuous veins of the pampiniform plexus of the spermatic cord. Dilatation of the pampiniform plexus larger than 1.5–2 cm in total diameter in adults (in resting state and in supine position) is considered as a varicocele. Correspondingly, in children, a diameter larger than 0.5 cm in prepuberty and larger than 0.8 cm in puberty is described as varicocele, respectively [1]. The veins of the pampiniform plexus normally range from 0.5 to 1.5 mm in diameter, and a dilatation greater than 2 mm in diameter is characteristic for varicocele.
The vast majority of the varicoceles are primary due to defective incompetent valves in the testicular vein. In contrast, the less common secondary varicoceles result from an increased pressure in the testicular veins caused by compression by an extrinsic mass, or obstruction.
Varicoceles are commonly located on the left side (85 %). This clear difference in predominance may be due to the shorter course of right testicular vein and its oblique insertion directly into the inferior vena cava, in contrast to the left testicular vein, which has a longer course and a right angle entry into the left renal vein.
Varicocele can be reliably diagnosed with ultrasound, which should be performed in both supine and standing positions including the Valsalva maneuvers (Figs. 16.5, 16.6, 16.7, 16.8 and 16.9). Power Doppler mode should be used to confirm flow in the varicocele, and there may even be flow reversal with the Valsalva maneuver, which is useful to grade the degree of reflux.
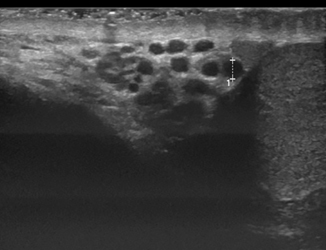
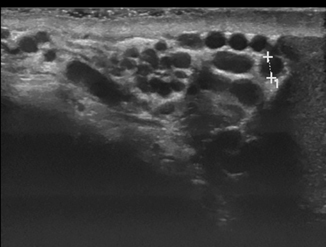
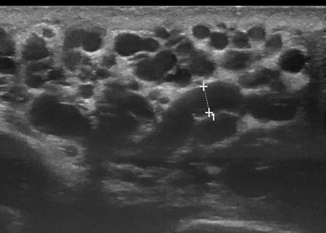
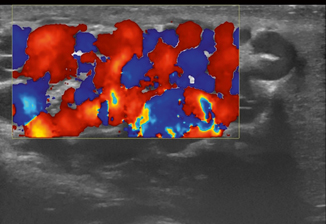

Fig. 16.5
Longitudinal scan of the pampiniform plexus in a 15-year-old boy with varicocele measuring the vein diameter in supine position of the patient

Fig. 16.6
Same patient as in Fig. 16.5 during the Valsalva maneuver resulting in moderate dilatation of the vessels


Fig. 16.9
Same patient as in Fig. 16.8 color Doppler ultrasound (CDU) image showed no further increase of the vein dilatation after 1 min of the Valsalva maneuver in standing position
In the literature, several grading systems for varicocele severity have been proposed.
The most used ultrasound grading system was proposed by Sarteschi [12, 13], grading the varicocele into five grades according to its length, the characteristics of the reflux, and changes during the Valsalva’s maneuver by CDU:
Grade 1 is characterized by non-dilated intrascrotal veins by grey-scale study, and prolonged reflux in the vessels in the inguinal canal during the Valsalva’s maneuver.
Grade 2 shows a prominent posterior varicosity at the upper pole of the testis and reflux into the supratesticular region during the CDU evaluation.
Grade 3 is characterized by major dilatation of the venous vessels in the supine position, and to the inferior pole of the testis in standing position only. CDU demonstrates reflux at the lower pole veins only during the Valsalva maneuver.
Grade 4 has dilated veins in an supine position. This enlargement of the veins increases in the standing position over time and during Valsalva’s maneuver. There is clear increase of the venous reflux after the Valsalva’s maneuver. Hypotrophy of the testis is common at this stage.
Grade 5 shows venous dilatation even in a supine position. CDU demonstrates the presence of a baseline basal venous reflux without the Valsalva maneuver that does not further increase after the Valsalva maneuver (Table 16.1).
Score
Maximum vein diameter (mm)
< 2.5 0
0
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access