Head and Neck Imaging
Jerome A. Barakos
Derk D. Purcell
“Head and neck” is a term used collectively to describe the extracranial structures, including the sinonasal cavity, skull base, pharynx, oral cavity, larynx, neck, orbit, and temporal bone. The head and neck region encompasses a tremendous spectrum of tissues in a compact space, with almost every organ system represented, including the digestive, respiratory, nervous, osseous, and vascular systems. Because of this anatomic complexity, the head and neck region is an area approached with considerable trepidation. However, accurate assessment of this area can be accomplished by combining an understanding of the normal anatomy, with familiarity of the scope of pathological entities that may occur in this region. We will begin our discussion by considering lesions of the paranasal sinuses and nasal cavity. This will be followed by a review of the skull base, the deep spaces of the neck, the lymph nodes, the orbits, and finally congenital head and neck lesions.
Imaging Methods. Both multislice helical/spiral CT and MR can provide exquisite imaging of the normal and pathologic anatomy of the head and neck. Although each modality has advantages and disadvantages, the decision on whether to use CT versus MR for each individual case is often based on considering which technique the patient is more likely to tolerate. For example, if a patient has difficulty handling their oral secretions because of prior head and neck surgery, particularly following tracheotomy or partial glossectomy, they may have significant hardship lying still for the time required for MR scanning. In such cases, the rapid imaging time of CT is more likely to yield a study unmarred by motion artifact. Because calcification is better depicted with CT, this is the modality of choice when looking for obstructing salivary ductal calculi (sialoliths) or for the detection of fractures. A principal drawback with CT is the increasing concern of radiation exposure, especially in the pediatric and young adult population. However, in an older adult, especially with a known malignancy, the potential advantages of CT, including rapid scanning and reduced motion artifact should serve to outweigh any radiation exposure concerns. In contrast, MR provides outstanding sensitivity for the discrimination of soft tissues and often better demonstrates the full extent of pathology. At the same time, the superior tissue contrast discrimination of MR allows for enhanced diagnostic specificity. The direct multiplanar capability of MR may also provide for improved evaluation of pathologic entities. For example, because of the horizontal orientation of the palate, floor of the mouth, and skull base, sagittal and coronal imaging are invaluable in optimally assessing these areas.
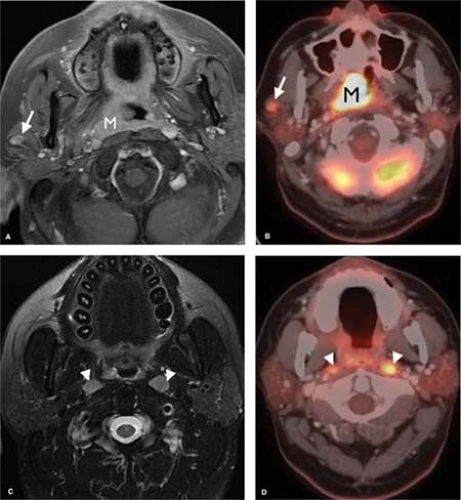
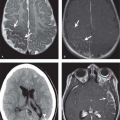
PET. The advent of PET imaging has had a profound effect on the evaluation and staging of head and neck malignancies. In combination with either MR or CT imaging, PET has greatly increased the sensitivity and specificity in the evaluation of primary as well as recurrent malignancies. PET is a functional imaging modality based upon the distribution of a glucose analogue radioisotope (18-F-fluorodeoxyglucose). Pathologic conditions that have an affinity for glucose will take up this isotope at a greater rate than normal surrounding tissues and thus be identifiable as areas of abnormality (Fig. 9.1).
Lesions found on PET scan are characterized by a standardized uptake value (SUV). The SUV refers to the relative radioactivity of a particular lesion when standardized to the injection dose and adjusted for body weight. As a result, the SUV is an absolute value that can be compared from patient to patient and exam to exam. In general, an SUV of greater than 3 is considered pathologic, but there are many caveats. A wide variety of nonmalignant conditions may give rise to an elevated SUV, most notably infection and postoperative changes. Additionally, some neoplasms have poor glucose affinity, resulting in a low SUV. PET alone may be highly sensitive, but it is not very specific. The true benefit of PET is realized when its physiologic/functional information is combined with the high-spatial-resolution morphologic information of CT and/or MR. In summary, combining PET findings with CT and MR, results in a marked increase in sensitivity and specificity, making this combination a powerful diagnostic tool.
Paranasal Sinuses and Nasal Cavity
Sinusitis. Inflammatory disease is the most common pathology involving the paranasal sinuses and nasal cavity. Mild mucosal thickening, primarily within the maxillary and ethmoid
sinuses, is common, even in asymptomatic individuals. In contrast, acute sinusitis is characterized by the presence of air–fluid levels or foamy-appearing sinus secretions and is typically caused by a viral upper respiratory tract infection (Fig. 9.2). In chronic sinusitis, changes include mucoperiosteal thickening as well as osseous thickening of the sinus walls. Soft tissue findings suggestive of sinusitis are best detected on T2WIs, as they are often high in signal. An exception is chronic sinus secretions that have become so desiccated that they yield no signal on either T1 or T2WIs and may mimic an aerated sinus. These sinus concretions and the bony wall thickening associated with chronic sinusitis are most easily appreciated on CT. Similar findings of hypointense T1 and T2WI sinus opacification have also been described in chronic noninvasive aspergillus sinusitis and chronic allergic hypersensitivity aspergillus sinusitis.
sinuses, is common, even in asymptomatic individuals. In contrast, acute sinusitis is characterized by the presence of air–fluid levels or foamy-appearing sinus secretions and is typically caused by a viral upper respiratory tract infection (Fig. 9.2). In chronic sinusitis, changes include mucoperiosteal thickening as well as osseous thickening of the sinus walls. Soft tissue findings suggestive of sinusitis are best detected on T2WIs, as they are often high in signal. An exception is chronic sinus secretions that have become so desiccated that they yield no signal on either T1 or T2WIs and may mimic an aerated sinus. These sinus concretions and the bony wall thickening associated with chronic sinusitis are most easily appreciated on CT. Similar findings of hypointense T1 and T2WI sinus opacification have also been described in chronic noninvasive aspergillus sinusitis and chronic allergic hypersensitivity aspergillus sinusitis.
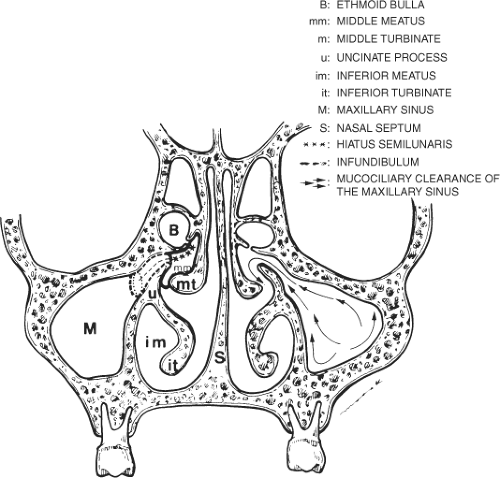
Endoscopic sinonasal surgery, used for the evaluation and treatment of inflammatory sinonasal disease, is being performed with increasing frequency. Direct coronal sinus CT provides exquisite definition of sinonasal anatomy and provides pre-endoscopic sinus assessment (Fig. 9.3). Knowledge of the anatomy of the lateral wall of the nasal cavity and routes of mucociliary drainage of the paranasal sinuses is critical to understanding patterns of inflammatory sinonasal disease. A major area of mucociliary drainage is the middle meatus, known as the ostiomeatal unit. It is important to note that disease limited to the infundibulum of the maxillary ostium will result in isolated obstruction of the maxillary sinus. In contrast, a lesion located in the hiatus semilunaris (middle meatus) results in combined obstruction of the ipsilateral maxillary sinus, anterior and middle ethmoid air cells, and the frontal sinus. This combined pattern of sinonasal disease has been described as the “ostiomeatal pattern” of obstruction. This pattern is significant because it indicates that one’s attention should be directed to identifying the offending lesion within the hiatus semilunaris, rather than simply describing the presence of diffuse sinus disease.
Several common complications are associated with sinusitis, including inflammatory polyps, mucous retention cysts, mucoceles, and most importantly cavernous sinus thrombosis.
Inflammatory Polyps. Chronic inflammation leads to mucosal hyperplasia, which results in mucosal redundancy and polyp formation. Most often these polyps blend imperceptibly with the mucoperiosteal thickening and cannot be clearly differentiated. When an antral polyp expands to the point where it prolapses through the sinus ostium, it is referred to as an antrochoanal polyp. Although these polyps may not
be associated with chronic sinusitis, they are similar to inflammatory polyps in that they represent areas of reactive mucosal thickening. Their characteristic appearance is that of a soft tissue mass extending from the maxillary sinus to fill the ipsilateral nasal cavity and nasopharynx. Often, the ostium of the maxillary sinus will be enlarged secondary to the mass effect of the polyp. The importance in recognizing such a lesion is that if it is surgically snared as if it were a nasal polyp, without regard for its antral stalk, it will recur.
be associated with chronic sinusitis, they are similar to inflammatory polyps in that they represent areas of reactive mucosal thickening. Their characteristic appearance is that of a soft tissue mass extending from the maxillary sinus to fill the ipsilateral nasal cavity and nasopharynx. Often, the ostium of the maxillary sinus will be enlarged secondary to the mass effect of the polyp. The importance in recognizing such a lesion is that if it is surgically snared as if it were a nasal polyp, without regard for its antral stalk, it will recur.
Mucous retention cysts simply represent obstructed mucous glands within the mucosal lining. These lesions have a characteristic rounded appearance, measuring one to several centimeters in diameter, with the maxillary sinus being most commonly involved. These lesions are commonly recognized in asymptomatic individuals.
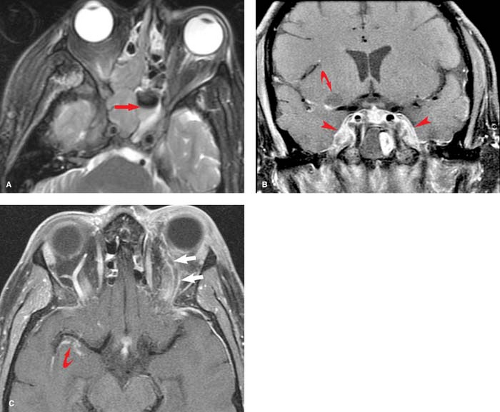
Mucocele is similar to a retention cyst, but instead of disease being confined to the single mucous gland, the lesion expands to the point where the entire sinus becomes obstructed. This typically occurs because of a mass obstructing the draining sinus ostium. The characteristic feature of a mucocele is frank expansion of the sinus with associated sinus wall bony thinning and remodeling. The frontal sinus is the sinus most commonly affected, but any sinus may be involved (Fig. 9.4). If the mucocele becomes infected, it demonstrates peripheral enhancement and is referred to as a mucopyocele.
Inverting Papilloma. A variety of papillomas occur within the nasal cavity, but most attention has focused on the inverting papilloma. These papillomas are named based on their histologic appearance. In this condition, the neoplastic nasal epithelium inverts and grows into the underlying mucosa. These papillomas are not believed to be associated with allergy or chronic infection because they are almost invariably unilateral in location. Inverting papillomas occur exclusively on the lateral nasal wall, centered on the hiatus semilunaris. Because of their increased association with squamous cell carcinoma, it is recommended that these lesions be surgically resected with wide mucosal margins.
Juvenile nasopharyngeal angiofibromas are typically seen in male adolescents presenting with epistaxis. The tumor arises from fibrovascular stroma of the nasal wall adjacent to the sphenopalatine foramen. This is a benign tumor that can be very locally aggressive. In an adolescent male presenting with a nasal mass and epistaxis, it is important to have a high clinical suspicion for this lesion, because life-threatening hemorrhage may result if a biopsy or limited resection is attempted. The tumor characteristically fills the nasopharynx and bows the posterior wall of the maxillary sinus forward. In fact, the retromaxillary pterygopalatine fossa location is a hallmark feature that should elicit this diagnosis for consideration. Juvenile nasopharyngeal angiofibromas enhance markedly with contrast administration, differentiating them from the rarer lymphangioma. Preoperatively, interventional radiology may play a role in embolization of these lesions, making them less vascular and facilitating surgical resection.
Malignancies. The tissues within the paranasal sinuses and nasal cavity that give rise to malignancies include squamous
epithelium, lymphoid tissue, and minor salivary glands. The corresponding malignancies are therefore squamous cell carcinoma, lymphoma, and minor salivary tumors. Because the entire upper aerodigestive tract is lined with squamous epithelium, it follows that squamous cell carcinoma is the most common malignancy (80% to 90%) of not only the paranasal sinuses and nasal cavity, but of the entire head and neck. Squamous cell carcinoma of the sinuses is often clinically silent until it is quite advanced. Early symptoms are usually related to obstructive sinusitis. Imaging findings consist of an opacified sinus with associated bony wall destruction. These findings are nonspecific and do not allow differentiation from non-Hodgkin lymphoma or a minor salivary gland malignancy. The presence of constitutional symptoms with prominent head and neck or systemic adenopathy suggests lymphoma, particularly in a child or young adult.
epithelium, lymphoid tissue, and minor salivary glands. The corresponding malignancies are therefore squamous cell carcinoma, lymphoma, and minor salivary tumors. Because the entire upper aerodigestive tract is lined with squamous epithelium, it follows that squamous cell carcinoma is the most common malignancy (80% to 90%) of not only the paranasal sinuses and nasal cavity, but of the entire head and neck. Squamous cell carcinoma of the sinuses is often clinically silent until it is quite advanced. Early symptoms are usually related to obstructive sinusitis. Imaging findings consist of an opacified sinus with associated bony wall destruction. These findings are nonspecific and do not allow differentiation from non-Hodgkin lymphoma or a minor salivary gland malignancy. The presence of constitutional symptoms with prominent head and neck or systemic adenopathy suggests lymphoma, particularly in a child or young adult.
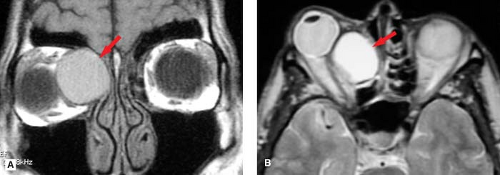 Figure 9.4. Sinus Mucocele. A. Coronal T1WI. B. Axial T2WI. Patient presented with proptosis, resulting from mass effect from an ethmoid sinus mucocele (arrows). A mucocele results from chronic obstruction of a paranasal sinus that becomes blocked and converted into a fluid-filled cyst. Over time this lesion may expand, eroding bone and resulting in proptosis. Differential diagnostic considerations would include a dermoid cyst, which would be characterized by the presence of fat (see Fig. 9.36). |
Minor salivary glands are dispersed throughout the upper aerodigestive tract but are most highly concentrated in the palate. Any of these minor salivary glands found throughout the head and neck, may give rise to salivary neoplasms. In contrast to parotid gland salivary neoplasms, the majority of which are benign, most minor salivary neoplasms are malignant. The most common salivary malignancies include adenoid cystic carcinoma, adenocarcinoma, and mucoepidermoid carcinoma.
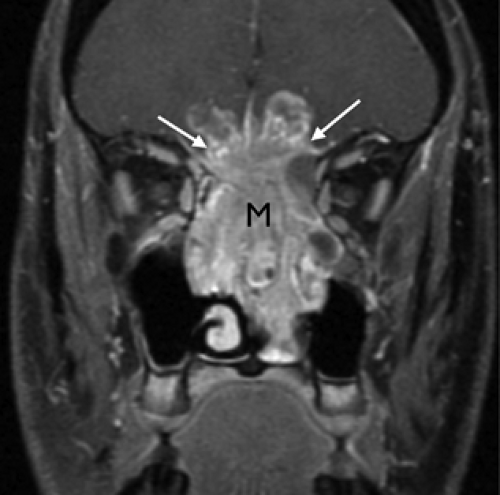
An esthesioneuroblastoma is an additional malignancy that should be mentioned when describing lesions of the nasal cavity. The esthesioneuroblastoma is a tumor that arises from the neurosensory receptor cells of the olfactory nerve and mucosa. Thus, this lesion may originate anywhere from the cribriform plate to the turbinates. This tumor is often quite destructive by the time of diagnosis and is found high within the nasal vault (Fig. 9.5). Involvement of the cribriform plate with extension into the anterior cranial fossa is not uncommon with esthesioneuroblastoma and should suggest this diagnosis.
In assessing the size and extent of sinonasal cavity pathology, it is often difficult to differentiate the offending lesion from associated obstructed sinus secretions. In such instances, fat-suppressed T2WI sequences are of value, because in general, sinus secretions will be brighter than the malignancy, which is often isointense with respect to muscle.
Skull Base
The skull base extends from the nose anteriorly to the occipital protuberance posteriorly and is composed of five bones: the ethmoid, sphenoid, occipital, temporal, and frontal bones. The skull base contains many foramina through which both vessels and nerves pass. Because the skull base has an undulating surface with a horizontal orientation, coronal or sagittal images are valuable in its evaluation.
Tumors of the Skull Base
Tumors may arise that are intrinsic to the skull base. Additionally, an extrinsic lesion may extend to involve the skull base from either above or below. Any lesion from the paranasal sinuses and nasal cavity already described, may extend to involve the skull base. Other lesions that may extend to involve the skull base include paragangliomas, neural sheath tumors (schwannoma and neurofibroma), and meningiomas. Although various primary malignant neoplasms of the skull base are described later, most malignant lesions of the skull base are metastatic in origin.
Primary malignant neoplasms are relatively uncommon, comprising only about 2% to 3% of skull base tumors. The three most common primary malignant tumors are chordoma, chondrosarcoma, and osteogenic sarcoma. Differentiating these lesions, especially chordomas from chondrosarcomas using both radiologic and histologic criteria can be difficult. Thus the anatomic location of these lesions proves useful in suggesting one lesion over another. Chordoma is a bone neoplasm that arises from remnants of the primitive notochord. Classically, this lesion will present as a destructive midline mass centered in the clivus. These tumors may be found anywhere along the craniospinal axis; typically 35% of lesions involve the clivus, 50% the sacrum, and 15% the vertebral bodies. Radiographically, this lesion is characterized as a midline destructive bony lesion with predilection for the sphenooccipital synchondrosis. On a sagittal image, the sphenooccipital synchondrosis is occasionally seen as a horizontal line in the midclivus, midway between sella and basion (tip of clivus). Chondrosarcomas are malignant tumors that develop from cartilage. Because the skull base is preformed in cartilage, there is a predilection for chondrosarcoma to involve the skull base. A preferred site of origin is parasellar in location, at the petroclival junction. Osteogenic sarcoma is typically the result of prior radiation therapy or malignant transformation of Paget disease.
Although a central destructive clival lesion is characteristic for chordoma and a paraclival destructive bony lesion is suggestive of chondrosarcoma, our differential diagnostic list includes several other bony lesions. The skull base, like any bone, may be affected by metastases, myeloma, plasmacytoma, fibrous dysplasia, and Paget disease. As with any bony lesion, CT helps to differentiate among these diagnostic possibilities. For example, fibrous dysplasia will reveal a smooth, ground-glass appearance on CT, while Paget disease will demonstrate trabecular coarsening, and neither of these conditions will reveal bony destruction.
Lesions of the jugular foramen are most commonly paragangliomas and are discussed under the heading “Carotid Space.” Paragangliomas arise from glomus cells derived from the embryonic neural crest, functioning as part of the sympathetic nervous system. As such, they may occur anywhere
along the sympathetic fibers of the head and neck, but those involving the skull base, specifically the jugular foramen are referred to as a glomus jugulare. These patients commonly present with pulsatile tinnitus and a conductive hearing loss. CT and MR may play complementary roles in evaluating these lesions. CT often demonstrates “moth-eaten” destruction of the bone surrounding the jugular fossa, with MR revealing the typical heterogeneous “salt-and-pepper” signal related to numerous flow voids. Malignant tumors are often indistinguishable from paragangliomas on CT, but most fail to demonstrate flow voids on MR. Other lesions of the jugular fossa include schwannomas (arising from cranial nerves IX to XI) and meningiomas. These lesions cause a smooth expansion of the jugular foramen with marked enhancement. Additionally, schwannomas may demonstrate cystic components.
along the sympathetic fibers of the head and neck, but those involving the skull base, specifically the jugular foramen are referred to as a glomus jugulare. These patients commonly present with pulsatile tinnitus and a conductive hearing loss. CT and MR may play complementary roles in evaluating these lesions. CT often demonstrates “moth-eaten” destruction of the bone surrounding the jugular fossa, with MR revealing the typical heterogeneous “salt-and-pepper” signal related to numerous flow voids. Malignant tumors are often indistinguishable from paragangliomas on CT, but most fail to demonstrate flow voids on MR. Other lesions of the jugular fossa include schwannomas (arising from cranial nerves IX to XI) and meningiomas. These lesions cause a smooth expansion of the jugular foramen with marked enhancement. Additionally, schwannomas may demonstrate cystic components.
Temporal Bone
Although a thorough discussion of the temporal bone is beyond the scope of this chapter, we will focus on some highlights. The most common diseases involving the temporal bone are inflammatory in nature and include cholesteatomas. Eustachian tube dysfunction with resultant decreased intratympanic pressure is believed to be the principal defect responsible for inflammatory disease of the middle ear and mastoid.
Cholesteatoma is an epidermoid cyst composed of desquamating stratified squamous epithelium. These cysts enlarge because of the progressive accumulation of epithelial debris within their lumen. They may be either congenital (2%) or acquired (98%). Congenital cholesteatomas originate from epithelial rests within or adjacent to the temporal bone. Acquired cholesteatomas originate from the stratified squamous epithelium of the tympanic membrane. These begin as localized tympanic membrane retraction pockets. The diagnosis of a cholesteatoma is based on the detection of a soft tissue mass within the middle ear cavity, typically with associated bony erosion. The superior portion of the tympanic membrane (pars flaccida) retracts easily and is the most common site for formation of an acquired cholesteatoma. Cholesteatomas arising in this area originate within the Prussak space (superior recess of the tympanic membrane), which is located medial to the pars flaccida between the scutum and the neck of the malleus. Thus, a finding of soft tissue in this region with subtle erosion of the scutum and medial displacement of the ossicles is characteristic of a cholesteatoma. Note that when fluid or inflammatory pathology is present, such as with otitis media, these changes cannot be differentiated from cholesteatoma because they have similar densities.
Although most cholesteatomas can be easily diagnosed otoscopically, the clinician cannot judge the size and full extent of the lesion. As a result, CT plays an important role in determining the size of the lesion, as well as the status of the ossicles, the labyrinth, the tegmen, and the facial nerve. MR has a limited role in the evaluation of erosive lesions of the temporal bone, because poor visualization of osseous landmarks limits localization of the process and it gives little information concerning the status of the ossicles and other bony structures. However cholesteatomas often reveal restricted diffusion (high signal) on diffusion-weighted echo-planar imaging (DW-EPI). Thus MR with diffusion imaging may provide complementary value in the initial diagnosis, or utility in the evaluation of residual or recurrent cholesteatoma.
Cholesterol granuloma, also known as giant cholesterol cyst, is a type of granulation tissue that may involve the petrous apex. These lesions represent petrous apex air cells that have become partially obstructed and are filled with cholesterol debris and hemorrhagic fluid. Because of their hemorrhagic components, these lesions are characterized by high signal on both T1WIs and T2WIs. When faced with an opacified petrous apex, differential diagnostic considerations include retained fluid secretions (parallels signal intensity of fluid, dark T1, bright T2, and no enhancement); petrous apicitis (parallels signal intensity of abscess, dark T1, bright T2, and ring enhancement); and nonaerated petrous apex (parallels signal intensity of fatty bone marrow, bright T1, dark T2, and no enhancement).
Suprahyoid Head and Neck
When a patient presents with a head and neck mass, the age of presentation is an important consideration when establishing a differential diagnostic list. In the pediatric age group, the majority of lesions (>90%) will be benign and consist of a variety of congenital or inflammatory entities (see “Congenital Lesions”). If a malignancy is encountered, it will most likely be a lymphoma (e.g., Burkitt lymphoma if rapid growth is noted) or rhabdomyosarcoma. In sharp contrast, when an adult presents with a head and neck mass (excluding thyroid lesions), the vast majority of lesions (>90%) will be malignant (Fig. 9.6). In the younger adult (20 to 40 years), the most common malignancy will be lymphoma, and in adults older than 40 years, the most common neck mass will be nodal metastases.
The suprahyoid head and neck is traditionally divided into compartments that include the nasopharynx, oropharynx, and oral cavity. An understanding of the division between these spaces is essential to accurately determine and describe the full extent of mucosal lesions.
The term nasopharynx is frequently misused as a nonspecific term to describe any area in the upper aerodigestive tract. In fact, the nasopharynx refers to a very specific portion of the pharynx. The nasopharynx lies above the oropharynx and is divided from the oropharynx by a horizontal line drawn along the hard and soft palates. Posteriorly the nasopharynx is bounded by the pharyngeal constrictor muscles, and anteriorly it is bounded by the nasal cavity at the nasal choana (paired funnel-shaped openings between the nasal cavity and the nasopharynx). Below the hard palate lie the oral cavity and oropharynx. These two areas are divided by a ring of structures that includes the circumvallate papillae (located along the posterior aspect of the tongue), the tonsillar pillars, and the soft palate.
These traditional compartments (nasopharynx, oropharynx, and oral cavity) are important for describing the spread of superficial, mucosa-based lesions. In contrast to this division, multiple facial planes divide the deep head and neck into spaces that form true compartments. It is important to realize that these deep spaces are unrelated to the traditional division of the head and neck and traverse the neck without regard to the traditional divisions. Therefore, when describing deep head and neck lesions, the traditional pharyngeal subdivisions are of limited value. Most radiologists have adapted a spatial approach to the head and neck, described as follows and popularized by Dr. Ric Harnsberger.
The deep anatomy of the head and neck is subdivided by layers of the deep cervical fascia into the following spaces: (1) superficial mucosal, (2) parapharyngeal, (3) carotid, (4) parotid, (5) masticator, (6) retropharyngeal, and (7) prevertebral. When evaluating a patient with pathology in the deep head and neck, it is important to determine within which space the pathology lies. Because only a limited number of structures are located within each compartment, these are the structures from which pathology will arise. Therefore, only specific pathology will be found within these separate fascial spaces, markedly limiting the differential diagnosis. For example, the principal structures within the parotid space are
the parotid gland and parotid lymph nodes. Consequently, if a parotid space mass is identified, the diagnosis is primarily limited to either a parotid tumor or nodal disease. Each of these seven spaces will be reviewed in detail (Table 9.1). Note that although this spatial division is popular with radiologists, surgeons and otolaryngologists occasionally use different terms, e.g., “retrostyloid space” instead of “carotid space.”
the parotid gland and parotid lymph nodes. Consequently, if a parotid space mass is identified, the diagnosis is primarily limited to either a parotid tumor or nodal disease. Each of these seven spaces will be reviewed in detail (Table 9.1). Note that although this spatial division is popular with radiologists, surgeons and otolaryngologists occasionally use different terms, e.g., “retrostyloid space” instead of “carotid space.”
Superficial Mucosal Space
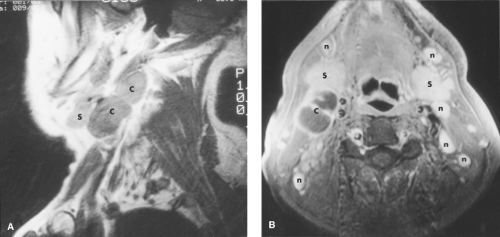
The superficial mucosal space includes all structures on the airway side of the pharyngobasilar fascia. The principal constituent of this space is the mucosa of the upper aerodigestive tract, which consists of squamous epithelium, submucosal lymphatics, and hundreds of minor salivary glands. The pharyngobasilar fascia represents the superior aponeurosis of the superior pharyngeal constrictor muscle, which inserts into the skull base. This tough fascia separates the mucosal space from the surrounding parapharyngeal space. Lesions originating within the superficial mucosal space may invade deep to the mucosal surface, resulting first in lateral displacement and then obliteration of the parapharyngeal space. However, many early lesions that begin within the mucosal space present as only mild mucosal irregularities or asymmetries (Fig. 9.7). This space is easily evaluated by the clinician and thus the radiologist should have a low threshold for suggesting the presence of abnormalities within this space. In children, there is frequently prominent adenoidal tissue that fills the nasopharynx. Even in adults, following a recent upper respiratory infection, prominent symmetrical mucosal tissue may be noted; this is of little concern as long as there is no invasion of deep facial places and no associated adenopathy (Fig. 9.8).
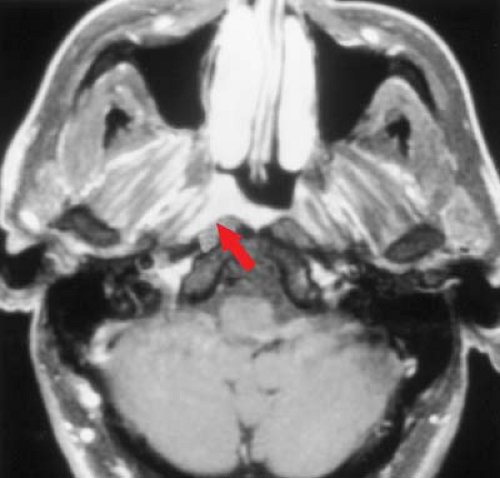
Benign Lesions. The most common benign lesions arising in the mucosal space are Tornwaldt cysts and lesions related to minor salivary gland tissue. Tornwaldt cysts are sharply marginated and are found in the midline with high signal intensity on T2WIs (Fig. 9.9). They are believed to be remnants of notochordal tissue aberrantly located in the nasopharynx and have an incidence of approximately 1% to 2% in normal patients. Lesions arising from minor salivary glands include retention cysts and benign neoplasms. Retention cysts represent obstructed glands similar to those found within the
paranasal sinuses. The most common benign neoplasm is the benign mixed-cell tumor (pleomorphic adenoma). Both of these lesions present as well-circumscribed, rounded lesions that have high signal intensity on T2WIs.
paranasal sinuses. The most common benign neoplasm is the benign mixed-cell tumor (pleomorphic adenoma). Both of these lesions present as well-circumscribed, rounded lesions that have high signal intensity on T2WIs.
Table 9.1 Deep Compartments of the Head and Neck | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Malignant Lesions. The most common malignant neoplasms of the mucosal space are squamous cell carcinoma, non–Hodgkin lymphoma, and minor salivary gland malignancies; of these, squamous cell carcinoma is by far the most common. Unfortunately, these malignancies all appear similar on CT and MR. Initially there is mass effect, often associated with lateral compression or obliteration of the parapharyngeal space, followed by invasion of the skull base. An early triad of radiographic findings consists of (1) superficial nasopharyngeal mucosal asymmetry, (2) ipsilateral retropharyngeal adenopathy, and (3) mastoid opacification. Mastoid opacification is an important early warning sign (Fig. 9.10). Mastoid opacification is easily detected on T2WIs and suggests potential dysfunction of the eustachian tube, frequently the result of tumor infiltration of the tensor veli palatini muscles. This finding directs the radiologist to carefully evaluate the mucosa of the nasopharynx. Note that both the nasopharynx and the mastoid air cells are included on every head CT and MR scan, and these areas should not be overlooked on routine head imaging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree