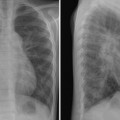
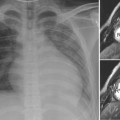
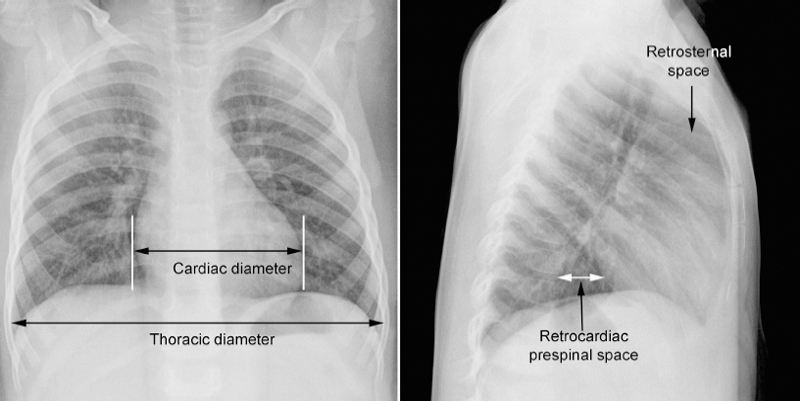
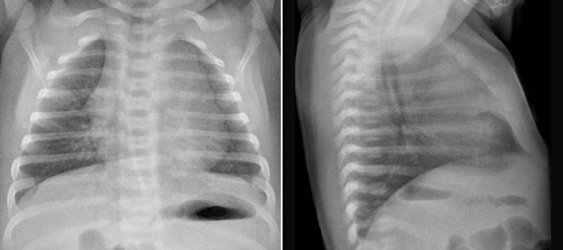
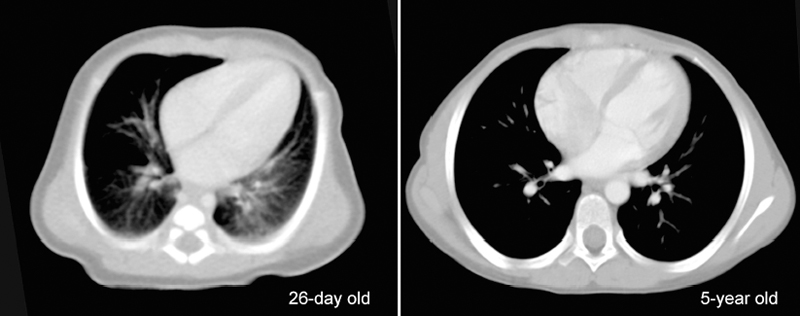
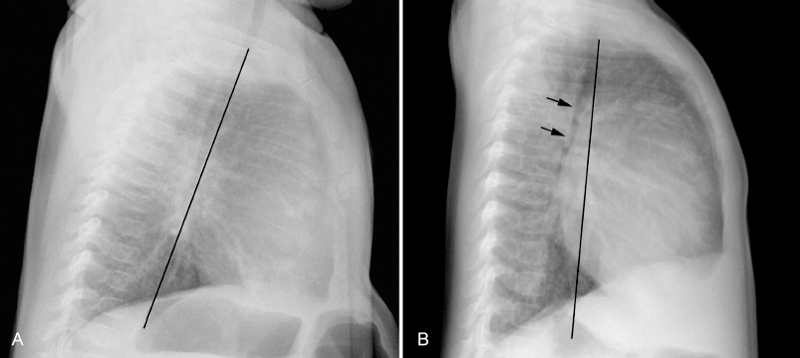
8 Heart Size, Overall Configuration, and Specific Chamber Enlargement The third step of the chest radiographic interpretation is to assess the overall heart size and configuration, and search for signs of specific chamber enlargement. For example, this part of the radiographic report can read as follows: “The heart is enlarged with a cardiothoracic ratio of 70% and shows an egg-on-side configuration. There are signs of right atrial enlargement that include increased height and outward bulge of the right atrial segment of the right cardiac contour on the frontal chest radiograph.” The heart size is estimated by comparing the transverse diameter of the cardiac silhouette with the transverse diameter of the thoracic cage seen on the frontal radiograph. It can be estimated either qualitatively or by calculating the cardiothoracic (CT) ratio as a percentage (Fig. 8.1). On a well-taken straight posteroanterior view, the CT ratio is less than 50% in normal adults and large children. The CT ratio can be up to 60% in newborns and diminishes to 50% within a year or two (Fig. 8.2).1 Assessment of the heart size may be difficult in young infants with a large thymus that overlies the heart on the frontal radiograph. The increased CT ratio in infants is partly due to a more circular configuration of the thoracic cage as compared with the adult chest (Fig. 8.3). In the first few days of life, volume overload secondary to excessive cord stripping may result in a transient increase in heart size. The lateral radiograph can also be used for heart size assessment. The tracheal air column is a useful landmark. Normally the posterior margin of the cardiac silhouette does not extend beyond the line that is drawn downward along the anterior wall of the trachea (Fig. 8.4A).2 The heart can be regarded as enlarged if its posterior part is seen beyond this line with or without evidence of posterior displacement of a bronchus or bronchi (Fig. 8.4B). An enlarged heart encroaches on the retrosternal precardiac or retrocardiac prespinal clear space. It should be noted, however, that the CT ratio on the lateral view is significantly affected by the depth of the thorax. Fig. 8.1 Normal posteroanterior and lateral radiographs of the chest from a 4-year-old child. Cardiothoracic ratio is the ratio between the transverse diameter of the cardiac silhouette and the maximum inner diameter of the thoracic cage seen on the frontal chest radiograph. It is important to be familiar with the extent of the normal retrosternal and retrocardiac prespinal spaces. Fig. 8.2 Normal posteroanterior and lateral chest radiographs from a 16-day-old neonate. The cardiothoracic ratio is larger than that is seen in Fig. 8.1. The normal thymus overlies the upper part of the heart, showing a sail sign on the right side and multiple thymic waves on the left side. The retrosternal space is filled with the thymus. The frontal chest radiograph is obtained with a slight caudal angulation, resulting in a lordotic view. Fig. 8.3 Difference in the shape of the thoracic cage between a neonate and a young child. The neonate shows a more circular configuration of the thoracic cage when compared with a 5-year-old child. Fig. 8.4 (A) Lateral chest radiographs from a normal infant and (B) from an infant with a ventricular septal defect. Lines are drawn along the anterior wall of the trachea and extended downward. The posterior margin of the normal heart in A does not extend beyond this limit, whereas the enlarged heart with a ventricular septal defect in B shows a prominent posterior margin bulging beyond the line. The bronchi (arrows) are displaced backward by the enlarged heart. Fig. 8.5 Right and left posterior oblique chest radiographs obtained from a newborn with levocardia. The cardiac silhouette is small on the right posterior oblique view (left panel) because the short axis of the heart is projected toward the screen, whereas it is larger on the left posterior oblique view (middle panel) because the long axis of the heart is projected toward the screen. Right panel shows that the right posterior oblique (RPO) and left anterior oblique (LAO) views project the short axis of the heart toward the screen, whereas the left posterior oblique (LPO) and right anterior oblique (RAO) views project the long axis of the heart toward the screen. In assessing heart size, it should be taken into consideration that the heart size can appear larger or smaller than it really is depending on the degree of inspiration and the radiographic projection. The transverse diameter of the cardiac silhouette becomes large with a high diaphragmatic position that can be due to incomplete inspiration, obesity, or abdominal pathology. The cardiac silhouette is more magnified on the anteroposterior view than on the posteroanterior view because the heart is located in the anterior part of the thorax. Subtle oblique projection may cause a significant difference in heart size and configuration. The heart in levocardia casts a smaller silhouette in the left anterior or right posterior oblique view than in a straight frontal view, whereas it casts a larger silhouette in the right anterior or left posterior oblique view (Fig. 8.5). The shape of the thoracic cage also influences the cardiac silhouette seen on the frontal radiograph. The heart appears large with an increased CT ratio in individuals who have a small overall thoracic cage or a circular thorax configuration as in newborns. The heart can be compressed and therefore casts a larger silhouette in a frontal radiograph, if the thorax has a decreased depth as in pectus excavatum and straight back syndrome or flat chest. The CT ratio may be less than 50% when there is overinflation of the lungs. Although CT ratio is more objective in assessing the heart size, subjective grading into borderline, mild but obvious, moderate, and severe cardiomegaly is more practical. It should be emphasized that the heart may maintain its normal size on radiographs until it is significantly enlarged (Figs. 8.6, 9.7A). Changes in overall heart size and configuration on radiographs are rather insensitive parameters of disease processes. Heart diseases can be grouped into two categories according to the loading condition: those with volume overload and those with pressure overload (Table 8.1). Isolated volume overload conditions include congenital heart diseases with left-to-right or left-to-left shunts, congenital and acquired lesions with valvular regurgitation, ventricular failure with stagnation of blood volume, large extracardiac vascular malformations such as vein of Galen malformation and hepatic hemangioendothelioma, and high cardiac output status such as severe anemia and thyrotoxicosis. Pressure overload conditions include congenital and acquired lesions with obstruction in the blood pathway that include outlet of a cardiac chamber, major arterial root, or peripheral vascular beds. For instance, valvular stenosis, coarctation of the aorta, peripheral pulmonary artery stenosis, obstructive pulmonary vascular disease, pulmonary venous obstruction, and systemic hypertension are all pressure overload lesions. Congenital heart diseases with right-to-left shunt, such as tetralogy of Fallot, are also pressure overload lesions. When there is a volume overload lesion, the overloaded chambers dilate with or without associated myocardial hypertrophy. Therefore, the radiographs show the signs of chamber enlargement that is proportional to the amount of the volume overload (Fig. 8.7A). The pressure overload lesions result in myocardial hypertrophy without dilatation of the cavity. As hypertrophy is both outward and inward, the external size of the hypertrophied chamber is only mildly increased whereas the cavity volume can be reduced. Therefore, hypertrophy results in only mild changes in chamber size on radiographs. Hypertrophy should be severe to cause significant enlargement of the cardiac silhouette. On the other hand, hypertrophy usually causes significant contour changes (Fig. 8.7B). When there is a pure right-to-left shunt without any valvular regurgitation, radiographic cardiomegaly is not seen because the intracardiac blood volume is reduced. Hypertrophy causes reduced compliance of the chamber and therefore impaired filling.
 Heart Size
Heart Size
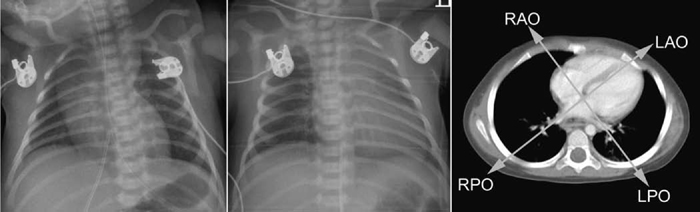
 Volume Versus Pressure Overload and Heart Size
Volume Versus Pressure Overload and Heart Size
| Volume Overload | Pressure Overload | |
| Pathology | Left-to-right shunt | Right-to-left shunt |
| Valvular regurgitation | Intracardiac or extracardiac obstructive lesions | |
| Ventricular failure | Pulmonary vein stenosis | |
| Large vascular malformation | Pulmonary hypertension | |
| Hyperdynamic circulation | Systemic hypertension | |
| Response of the involved cardiac chamber | Dilatation with or without hypertrophy | Hypertrophy |
| Compliance of the involved cardiac chamber | Mildly reduced | Severely reduced |
| Radiographic findings | Enlargement of the involved cardiac chambers proportional to volume overload | Contour changes with minimal changes in size |
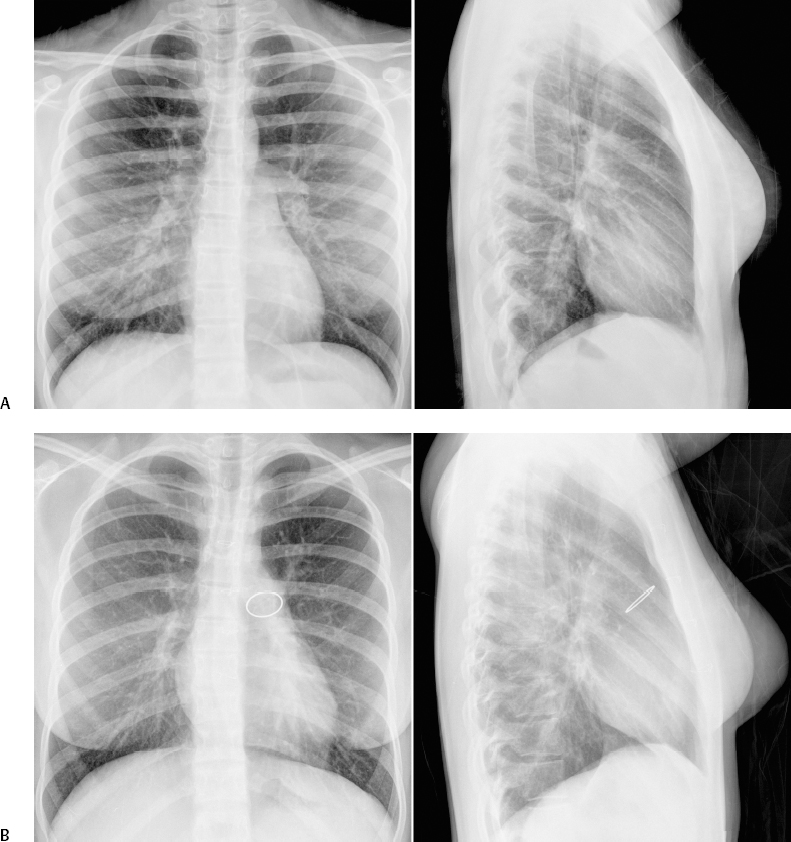
Fig. 8.6 Normal heart size with significant increase in ventricular volumes. (A) Atrial septal defect in a 15-year-old patient with pulmonary-to-systemic blood flow ratio (Qp/Qs) of 2.2 and right ventricular end-diastolic volume index of 151 mL/m2 of body surface area. (B) Moderately severe pulmonary regurgitation in a 16-year-old patient who previously underwent a Ross operation with an artificial pulmonary valve implantation for aortic stenosis. Magnetic resonance imaging (MRI) flow and ventricular volume assessment showed a pulmonary regurgitant fraction of 31% and right ventricular volume index of 119 mL/m2 of body surface area. The implanted pulmonary valve is calcified.
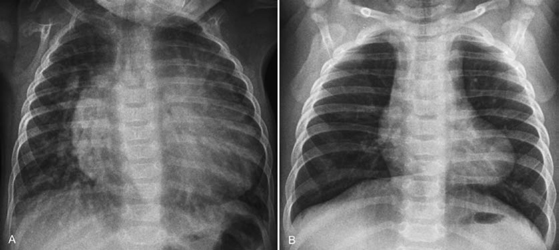
Fig. 8.7 Volume versus pressure overload lesions. (A) Volume overload lesion. Frontal chest radiograph from a 4-month-old infant with a large ventricular septal defect shows marked cardiomegaly and increased pulmonary vascularity. (B) Pressure overload lesion. Frontal chest radiograph from a 5-month-old infant with tetralogy of Fallot shows normal heart size. The cardiac apex is rounded and elevated. The pulmonary vascularity is mildly reduced.
 Overall Cardiac Configuration
Overall Cardiac Configuration
The overall cardiac configuration is often highly suggestive of a specific cardiac disease. One may be able to make a confident diagnosis of the specific disease based on the radiographic findings in conjunction with clinical symptoms and signs. Chest radiographs may provide clues to the diagnosis that may be missed by clinical or even echocardiographic investigation. This section introduces a few well-recognized named cardiac configurations, including boot-shaped heart, egg-on-side appearance, snowman appearance, triangular heart, cascade right heart border, and water-bottle appearance. Scimitar syndrome is discussed in Chapter 11.
Boot-Shaped Heart (Coeur en Sabot)
A boot-shaped heart is a well-known configuration of the heart that is most commonly seen in tetralogy of Fallot and tetralogy of Fallot with pulmonary atresia (pulmonary atresia with ventricular septal defect), and less commonly truncus arteriosus (Figs. 8.8, 8.9).3,4 The boot-shaped heart is characterized by a round and elevated cardiac apex and concave pulmonary arterial segment. Elevation and rounding of the cardiac apex are due to counterclockwise and leftward rotation of the heart as seen from front and below, respectively. As a result, both ventricular long axes lie more horizontally than normal, and the cardiac apical contour in the frontal view is formed by the elevated right or left ventricular apex. In extreme cases with a severely hypertrophied right ventricle, the ventricular apex can be higher than the base of the ventricles. The boot-shape with upturned cardiac apex is usually ascribed to concentric right ventricular hypertrophy. However, this appearance is not seen in patients with acquired right ventricular hypertrophy or in patients with severe pulmonary valve stenosis or pulmonary atresia and intact ventricular septum.4 The appearance, therefore, may represent the primary morphologic abnormality rather than right ventricular hypertrophy. The concave pulmonary arterial segment is due to hypoplasia of the right ventricular outflow tract and main pulmonary artery as well as an abnormal orientation of the main pulmonary artery coursing more rightward than normal. A boot-shaped heart can be seen without cardiomegaly in classic tetralogy of Fallot with predominant right-to-left shunt (Fig. 8.8A). A boot-shaped heart without significant cardiomegaly is also seen in pulmonary atresia with ventricular septal defect when the pulmonary blood supply is through a small patent ductus or stenotic systemic collateral arteries (Fig. 8.8B). When pulmonary atresia is associated with unobstructed collaterals, a boot-shaped heart is associated with dilated left-sided chambers, causing an enlarged cardiac silhouette (Fig. 8.9A). A large boot-shaped heart is also seen in truncus arteriosus (Fig. 8.9B).
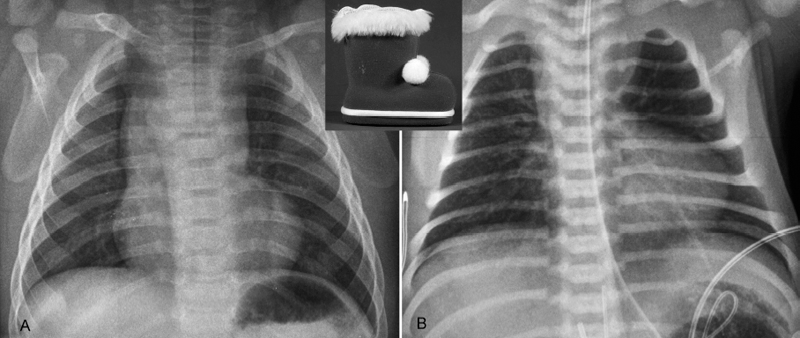
Fig. 8.8 Examples of small boots. (A) Tetralogy of Fallot in a 4-month-old infant. (B) Tetralogy of Fallot with pulmonary atresia and patent ductus arteriosus in a newborn. Both cases show an upturned and rounded cardiac apex. A large thymus hides the concave pulmonary arterial segment in A. The concave pulmonary arterial segment of the left heart border is uncovered in B because of thymic hypoplasia. This patient had microdeletion of chromosome 22q11.
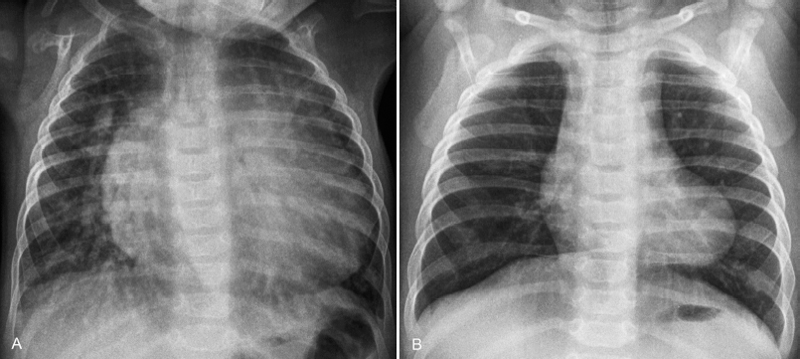
Fig. 8.9 Examples of large boots. (A) Pulmonary atresia with ventricular septal defect and multiple major aortopulmonary collateral arteries in a 14-year-old patient. The left lung is supplied by larger collateral arteries. (B) Truncus arteriosus in a 3-year-old patient. Both cases show a large boot-shaped heart. Cardiomegaly in both cases is due to volume overload with increased pulmonary blood flow.
Egg-on-Side Appearance
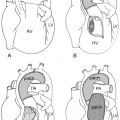
An egg-on-side appearance is classically seen in complete transposition of the great arteries (Fig. 8.10A).3 This configuration is mainly due to rightward displacement of the right ventricular outflow tract. Normally the right ventricular outflow tract is a left-sided structure and contributes to the mild convexity of the left midcardiac contour below the pulmonary arterial segment in the frontal view, although it is not border forming (Fig. 8.11, left panel). Therefore, the right ventricular outflow tract is partly responsible for the bulk of the left upper heart border. In complete transposition, the right ventricular outflow tract is a right-sided structure as it supports the ascending aorta that is located anterior to and rightward of the pulmonary trunk (Fig. 8.11, right panel). As a consequence, the left upper cardiac contour is flattened in complete transposition. The egg-on-side appearance may not be obvious on the first day of life when a large thymus obscures the cardiac contour. However, the cardiac contour is usually unveiled within a day or a few days as the thymus becomes smaller in coping with a postnatal critical condition. A similar feature is also seen in the so-called Taussig-Bing malformation, in which a double outlet right ventricle is associated with a subpulmonary ventricular septal defect (Fig. 8.10B). Complete transposition often shows a narrow superior mediastinum because of a parallel anteroposterior relationship of the great arteries.
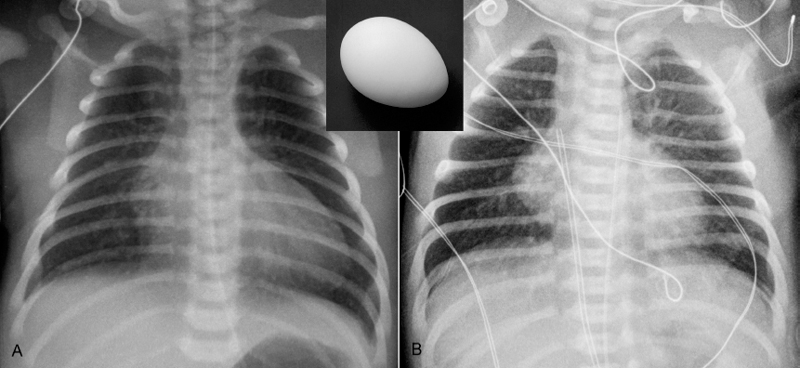
Fig. 8.10 Egg-on-side appearance. (A) Uncomplicated complete transposition of the great arteries in a 3-day-old neonate. (B) Double outlet right ventricle with subpulmonary ventricular septal defect and overriding pulmonary valve (so-called Taussig-Bing malformation) in a 2-day-old neonate. Both patients show a flattened left heart border below the pulmonary arterial segment. As shown in Fig. 8.11, this configuration in these two cases is due to rightward displacement of the right ventricular outflow tract. In both conditions, the great arteries have a parallel course with an anteroposterior relationship, showing a narrow superior mediastinum on the frontal view, in the majority of cases. The narrow vascular pedicle results from involuted thymus due to postnatal stress.
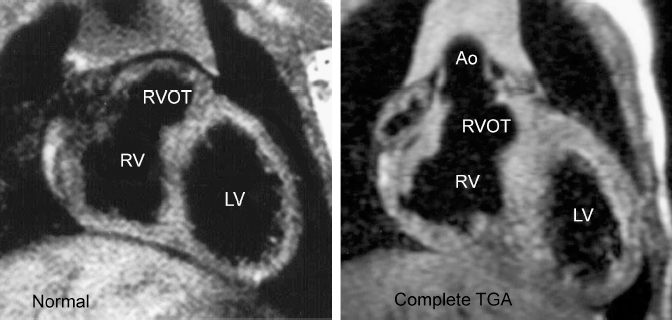
Fig. 8.11 Why the egg-on-side appearance in complete transposition of the great arteries (TGA)? Coronal T1-weighted MR images show a different location of the right ventricular outflow tract. Normally, the right ventricular outflow tract is located on the left connecting with the left-sided pulmonary trunk and forms a bulk underneath the left atrial appendage segment of the left heart border. In complete transposition (TGA), the right ventricular outflow tract is displaced rightward and the left upper heart border is flat. Ao, ascending aorta; LV, left ventricle; RV, right ventricle; RVOT, right ventricular outflow tract.
Truncus arteriosus may also show an egg-on-side appearance (Fig. 8.12A). In truncus arteriosus, a concave pulmonary arterial segment on the frontal view is due to absence of a pulmonary outflow tract. As in complete transposition, the thymus can be small due to a poor clinical condition. In addition, the thymus can be small or absent in association with DiGeorge or chromosome 22q11 microdeletion syndrome. In contrast to complete transposition, truncus arteriosus is associated with more significant cardiac enlargement, and typically there is increased pulmonary vascularity from the newborn period.
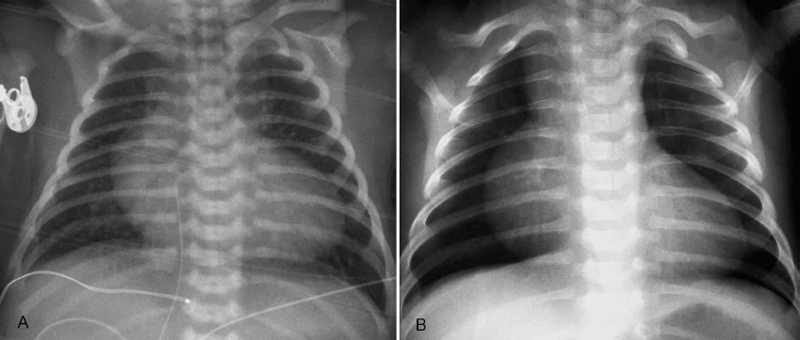
Fig. 8.12 Egg-on-side appearance in newborns with (A) truncus arteriosus and (B) pulmonary atresia with intact ventricular septum. In truncus arteriosus, the right ventricular outflow tract connects to the common arterial trunk. The left upper heart border is flat because there is no right ventricular outflow tract leading to the pulmonary artery. In pulmonary atresia with intact ventricular septum, the right ventricular outflow tract is hypoplastic, whereas the right atrium is dilated because of tricuspid regurgitation.
The egg-on-side appearance is also seen in pulmonary atresia with intact ventricular septum (Fig. 8.12B). The “egg” in this condition is typically large. The blunt side of the egg opposite to the apex is formed by an enlarged right atrium. The left upper heart border is flat because the right ventricular outflow tract is underdeveloped. The superior mediastinum is narrow because of the small size of the pulmonary arterial trunk and a small thymus. The pulmonary vascularity is typically diminished. A large heart with an egg-on-side appearance, right atrial enlargement, and decreased pulmonary vascularity in a cyanotic newborn is typical of pulmonary atresia or critical pulmonary stenosis with intact ventricular septum.
Snowman or Figure-of-8 Configuration
When it is seen with increased pulmonary vascularity, a snowman or figure-of-8 configuration is highly suggestive of total anomalous pulmonary venous connection to the innominate vein (Fig. 8.13A). The upper part is formed by the vertical vein that drains the pulmonary venous return on the left and the dilated superior vena cava on the right. The lower part is formed by the enlarged heart. However, this well-known sign is rarely apparent in infancy and therefore rarely encountered in developed countries, as a surgical correction is typically undertaken in the early neonatal period.5,6 As a caution particularly in infancy, a normal thymus can be globular in shape, and the cardiothymic silhouette can be similar to that of total anomalous pulmonary venous connection to the innominate vein on the frontal radiograph. On the lateral radiograph, a vertical band-like density can be seen along the anterior aspect of the trachea.5 We have experienced similar findings in a patient with a severe form of cor triatriatum, with the proximal chamber of the left atrium decompressed to the left innominate vein through a channel called a levoatriocardinal vein (Fig. 8.13B).7
Triangular Heart
The triangular cardiac silhouette is characterized by a straight left heart border (Fig. 8.14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree