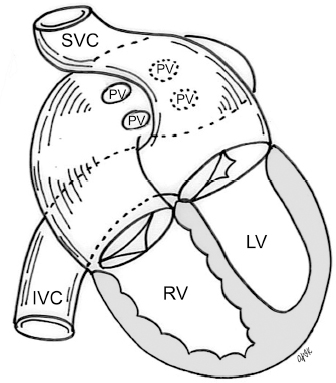
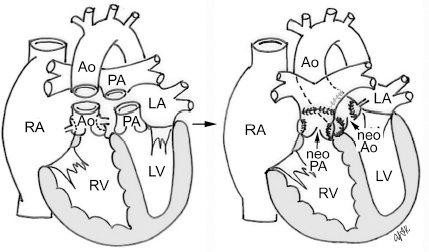
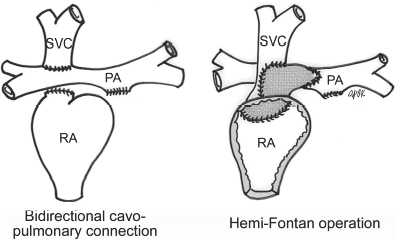
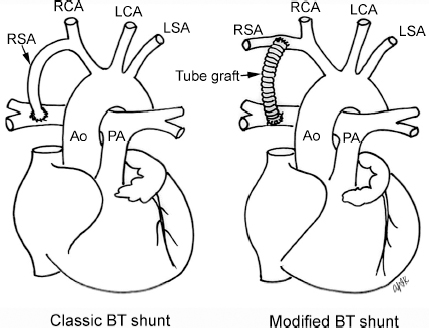
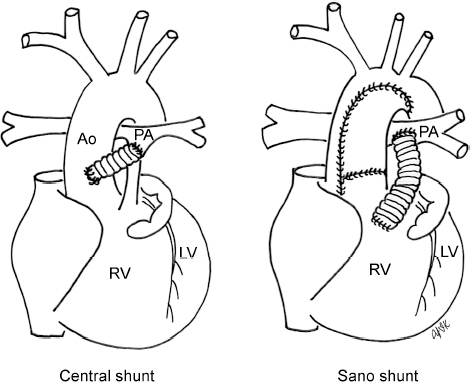
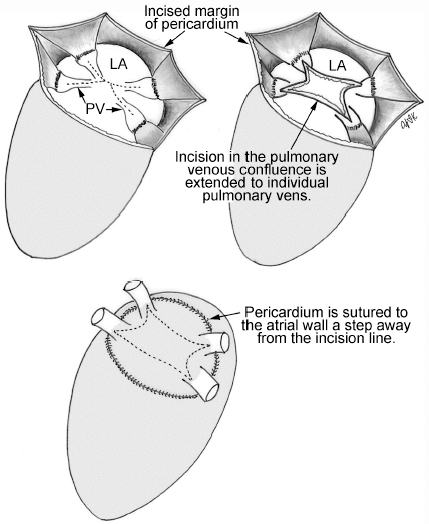
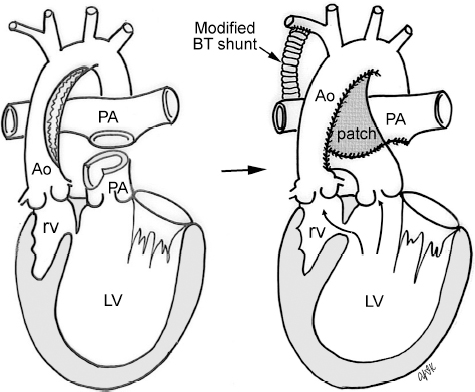
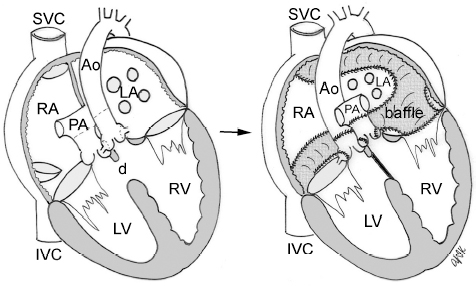
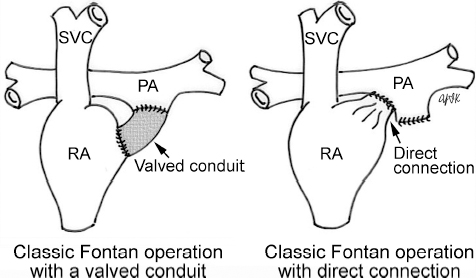
4 Glossary of Pediatric Cardiovascular Surgical Procedures As most congenital heart diseases are primarily structural defects, a surgical correction is required in the majority of the patients with a major defect. Surgery is performed when other forms of treatment cannot maintain adequate circulation or when the structural defect may result in damage to the heart, lungs, or other organs. Since Dr. Robert E. Gross performed the first surgical ligation of a patent ductus arteriosus in 1938,1 a number of surgical procedures have been introduced for corrective or palliative treatment of various congenital heart diseases. Many physicians who interpret plain radiographs for patients with congenital heart disease are not as familiar with various cardiovascular surgical procedures as they should be. In this chapter, we intend to overcome this gap by providing an overview of congenital cardiac surgery and various associated surgical procedures using a glossary format. Atrial Switch Operation Complete transposition of the great arteries requires switching of the abnormal circulation at one of three cardiac segment levels: the atrial, ventricular, or great arterial. Prior to the 1990s, the atrial switch operation had been the gold standard operation for complete transposition with intact ventricular septum or restrictive ventricular septal defect. Currently, the atrial switch has almost completely been replaced by the arterial switch operation in North American centers. Nevertheless, a large population of patients who underwent atrial switch procedures prior to the development of the arterial switch procedure are now adults and frequently present with long-term complications of the atrial switch including systemic venous baffle obstruction and systemic ventricular failure. Fig. 4.1 Atrial switch operation. RV, right ventricle; LV, left ventricle; PV, pulmonary vein; SVC, superior vena cava; IVC, inferior vena cava. An atrial switch operation consists of routing the systemic venous return to the left ventricle through the mitral valve and the pulmonary venous return to the right ventricle through the tricuspid valve by using an intraatrial baffle (Fig. 4.1). In the Senning operation, the atrial septum and atrial wall are used to construct the baffle.2 In the Mustard operation, the atrial septum is removed and the baffle is constructed with autologous pericardium or synthetic material.3 Although the atrial switch operation establishes a physiologic correction of the circulation, it leaves the right ventricle to support the systemic circulation, which is not desirable in the long term. In long-term follow-up, right ventricular failure, tricuspid regurgitation, and arrhythmias are common.4 Today its use is limited to congenitally corrected transposition of the great arteries in which the atrial switch operation is performed in conjunction with an arterial switch operation (See Double-Switch Operation below). Arterial Switch Operation (of Jatene) Complete transposition of the great arteries is now ideally repaired by an arterial switch operation pioneered in 1954 by Dr. William Mustard of Canada.5 It was successfully performed for the first time in 1976 by Dr. Adib Jatene of Brazil.6 As this operation restores the normal anatomic arrangement of the circulation, it has become the procedure of choice when the anatomy is appropriate.4 Fig. 4.2 Arterial switch operation using Lecompte maneuver. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; Ao, ascending aorta; PA, pulmonary artery. The operation consists of transection of the great arteries above the sinuses and detachment of the coronary arteries along with a button of aortic sinus wall, followed by translocation of the dissected great arteries into their new positions and implantation of the coronary buttons to the neo-aorta (Fig. 4.2). Translocation of the great arteries that are anteroposteriorly related requires a Lecompte maneuver in which the transected pulmonary artery bifurcation is moved anterior to the aorta.7 When the great arteries are related side-by-side (as in the Taus—sig—Bing anomaly), the Lecompte maneuver may not be necessary. In both cases, aortic and pulmonary artery continuity is restored and the procedure is completed. Bidirectional Cavopulmonary (Bidirectional Glenn) Connection Bidirectional Cavopulmonary (Bidirectional Glenn) Connection (BCPC) is used as an intermediate-stage palliation en route to a Fontan operation for patients with functionally single ventricle physiology.8,9 It is performed as a primary procedure or as a second-stage operation following a prior systemic artery-to-pulmonary artery shunt or a pulmonary artery banding. BCPC consists of dissection of the superior vena cava at its junction with the atrium and end-to-side anastomosis of the dissected superior vena cava to the ipsilateral pulmonary artery (Fig. 4.3).8,9 In those patients with bilateral superior venae cavae, but without a bridging innominate vein, it is necessary to perform bilateral bidirectional cavopulmonary connections. It is usually performed at 3 to 6 months of age as a second-stage procedure. It may be performed earlier when it is performed as a primary surgery. Occasionally, it can be the final surgical procedure when high pulmonary vascular resistance does not allow a subsequent Fontan operation. This procedure has unique benefits. Compared with systemic artery-to-pulmonary arterial shunts, it avoids volume overload to the ventricle and prevents pulmonary vascular obstructive disease and distortion. In addition, the volume of shunt flow increases with somatic growth. As the child ages, however, the relative body mass shifts disproportionately to larger lower body growth and relatively less upper body growth. Consequently, the ratio of blood passing through the superior vena cava and the inferior vena cava decreases. Because pulmonary blood flow is determined by superior vena caval flow after a BCPC, patients tend to develop progressive, deepening cyanosis as they enter late childhood. The hemi-Fontan operation (right panel in Fig. 4.3) is a variation of BCPC in which the continuity of the superior vena cava and right atrium is maintained when the superior vena cava is connected to the adjacent pulmonary artery.10 A patch is sewn across the junction between the superior vena cava and the right atrium through the vertical incision extending along the medial aspect of the superior vena cava and the superior aspect of the right atrium. This allows easy excision of the patch at the time of completion of a Fontan operation and establishment of continuity between the inferior vena cava and the pulmonary arteries with a lateral tunnel procedure (See Fontan operation). Fig. 4.3 Bidirectional cavopulmonary connection and hemi-Fontan operation. SVC, superior vena cava; PA, pulmonary artery; RA, right atrium. Blalock—Taussig Shunt Blalock and Taussig introduced this landmark procedure in 1945 to treat patients with pulmonary stenosis or atresia by directing blood flow from a systemic artery to the pulmonary artery for oxygenation.11 The Blalock—Taussig (BT) shunt has been widely used as a temporary procedure en route to corrective or further palliative surgery. Today the systemic artery-to-pulmonary arterial shunt is most commonly used in neonates with functionally single ventricle physiology as an intermediate procedure prior to a bidirectional cavopulmonary connection. The original BT shunt, which is now called a “classic BT shunt,” consisted of an end-to-side anastomosis of the subclavian artery to the ipsilateral pulmonary artery (Fig. 4.4, left panel). Subsequently, various modifications have been introduced. The most commonly used modification is the modified BT shunt, in which an artificial tube graft with a diameter of 3 to 5 mm is interposed between the subclavian or common carotid artery and the ipsilateral pulmonary artery (Fig. 4.4, right panel).12 Fig. 4.4 Classic and modified Blalock—Taussig (BT) shunts. RSA, right subclavian artery; LSA, left subclavian artery; RCA, right coronary artery; LCA, left coronary artery; Ao, ascending aorta; PA, pulmonary artery. Direct connection of the aorta to the branch pulmonary arteries using a Potts shunt (between descending aorta and left pulmonary artery) or a Waterston shunt (between ascending aorta and right pulmonary artery) has been abandoned because of a high incidence of subsequent pulmonary hypertension and frequent pulmonary artery kinking/distortion along with preferential flow to one lung.13 The central shunt uses a short tube graft between the ascending aorta and main pulmonary artery (Fig. 4.5, left panel).14 This procedure is used mainly for small neonates and young infants especially when the branch pulmonary arteries are very small. The Mee shunt is a direct central end-to-side anastomosis of the confluent pulmonary artery to the ascending aorta.15 The Sano shunt is a right ventricle-to-pulmonary artery shunt using a tube graft (Fig. 4.5, right panel).16 The major advantage of this procedure is maintenance of adequate diastolic pressure for coronary arterial perfusion in contrast to other arterial shunt procedures where systemic-to-pulmonary arterial run-off causes reduced aortic diastolic pressure that may contribute to coronary insufficiency. Fig. 4.5 Central shunt and Sano shunt. RV, right ventricle; LV, left ventricle; Ao, ascending aorta; PA, pulmonary artery. Central Shunt (See Blalock—Taussig shunt) Cole’s Procedure (Sutureless Pulmonary Vein Repair) Surgery that involves pulmonary vein dissection is often complicated by subsequent stenosis of the suture sites on the repaired pulmonary veins. Cole’s procedure was introduced to prevent postoperative stenosis of the pulmonary veins by avoiding direct suture of the pulmonary veins (Fig. 4.6).17 Fig. 4.6 Cole’s procedure. LA, left atrium; PV, pulmonary veins. For patients with primary or postsurgical stenosis of pulmonary veins, the posterior wall of the left atrium and the pulmonary venous confluence are incised transversely. The incision on the pulmonary venous confluence is extended to the individual pulmonary veins. The pericardium is then sutured to the atrial wall with a suture line kept away from the divided edge of the pulmonary veins. This suture line contains the pulmonary venous effluent in a “controlled bleed” while avoiding any direct suturing of the pulmonary veins. A similar technique can also be used for total anomalous pulmonary venous connection.17 Damus—Kaye—Stansel Operation In 1975, three different groups Damus—Kaye—Stansel (DKS) proposed a type of arterial repair for complex forms of complete transposition of the great arteries without coronary artery transfer.18–21 The basic concept of this operation is to use the pulmonary and aortic valves as a dual source of systemic cardiac output and to reconstruct the pulmonary outflow tract using a conduit. With increased experience with the classic arterial switch operation in patients with complex patterns of coronary artery anatomy, this approach for complex transposition or Taussig—Bing anomaly has been largely abandoned.22 Now, this procedure is typically used to relieve systemic ventricular outflow tract obstruction in a functionally single ventricle, such as tricuspid atresia or double-inlet left ventricle with subaortic stenosis.23 Fig. 4.7 Damus—Kaye—Stansel (DKS) operation. rv, rudimentary right ventricle; LV, left ventricle; Ao, ascending aorta; PA, pulmonary artery. This procedure consists of transection of the pulmonary artery, incision in the ascending aorta, end-to-side anastomosis of the proximal pulmonary arterial trunk to the ascending aorta, and augmentation of the anastomotic route by using an anterior patch (Fig. 4.7). The aortic valve and ventricular septal defect can be closed or left open. Pulmonary blood flow is reestablished by use of a modified BT or bidirectional cavopulmonary shunt depending on the patient’s age and the pulmonary vascular anatomy and resistance. Double-Switch Operation The standard surgical approach to congenitally corrected transposition of the great arteries is basically to treat the individual-associated abnormalities, such as repair or replacement of the dysplastic tricuspid valve, relief of pulmonary stenosis, and closure of a ventricular septal defect. However, the right ventricle may fail after a standard surgery because it supports the systemic circulation. Fig. 4.8 Double-switch operation. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; Ao, ascending aorta; PA, pulmonary artery; SVC, superior vena cava; IVC, inferior vena cava; d, ventricular septal defect. The double-switch operation consists of atrial and arterial switch procedures so that the left ventricle supports the systemic circulation (Fig. 4.8).24 The atrial septum is removed and the superior and inferior vena caval flow is directed to the right ventricle on the left side using an intraatrial baffle. Then, an arterial switch procedure is performed as described in the section for arterial switch operation. When corrected transposition is associated with a ventricular septal defect and pulmonary outflow tract obstruction, an atrial switch operation can be combined with a Rastelli-type of operation, which is called an Ilbawi operation.25,26 Both procedures certainly have a greater risk than other simple procedures, but a successful surgery results in better long-term outcome. Double switch is also needed in patients who have developed late complications of an atrial switch procedure for complete transposition. A previous atrial switch operation can be reversed with an arterial switch operation performed after a short period of left ventricular training by banding the main pulmonary artery. Fontan Operation A Fontan circuit is the final goal for all hearts with a functionally single ventricle. This includes double-inlet left or right ventricles, tricuspid atresia, pulmonary atresia with intact ventricular septum and hypoplastic left heart syndrome, as well as other rare malformations that do not allow biventricular repair. The principle of the Fontan operation is to commit the functional single ventricle to support the systemic circulation and to let blood flow passively through the lungs without being pumped by a ventricle. This procedure normalizes systemic oxygen saturation and eliminates volume overload of the functioning ventricle (Fig. 4.9). Fig. 4.9 Classic Fontan operation with or without valved conduit. SVC, superior vena cava; RA, right atrium; PA, pulmonary artery. The classic Fontan operation consisted of connecting the right atrium to the pulmonary artery and closing the atrial septal defect so that the systemic venous return bypasses the ventricles.27 Originally, a valved conduit was interposed between the right atrium and the pulmonary artery (Fig. 4.9, left panel).27 Subsequently, a direct valveless connection was preferred (Fig. 4.9, right panel). This classic procedure was often complicated by marked dilatation of the right atrium causing arrhythmias and formation of mural thrombi, as well as high systemic venous pressure resulting in anasarca, ascites, pleural effusion, and protein-losing enteropathy.
 Open-heart versus closed surgery. In general, intracardiac defects require an open-heart surgical procedure, which is done with cardiopulmonary bypass. Extracardiac defects involving the aorta, pulmonary vessels, or ductus arteriosus can be repaired by a closed procedure without cardiopulmonary bypass.
Open-heart versus closed surgery. In general, intracardiac defects require an open-heart surgical procedure, which is done with cardiopulmonary bypass. Extracardiac defects involving the aorta, pulmonary vessels, or ductus arteriosus can be repaired by a closed procedure without cardiopulmonary bypass.
 Complete versus palliative surgery. An ideal surgical procedure is a single-stage definitive corrective surgery. When the patient’s condition or the complexity of the cardiovascular pathology does not allow complete repair, palliative procedures are performed. Palliative procedures are aimed to alter the hemodynamic physiology of the particular defect so that the complications of the defect are treated, delayed, or prevented. Palliative procedures may be performed as preparatory measures for a later definitive surgery or end-stage palliative procedures.
Complete versus palliative surgery. An ideal surgical procedure is a single-stage definitive corrective surgery. When the patient’s condition or the complexity of the cardiovascular pathology does not allow complete repair, palliative procedures are performed. Palliative procedures are aimed to alter the hemodynamic physiology of the particular defect so that the complications of the defect are treated, delayed, or prevented. Palliative procedures may be performed as preparatory measures for a later definitive surgery or end-stage palliative procedures.
 Biventricular versus univentricular repair. Cardiovascular surgical procedures can be divided into biventricular and univentricular repairs. A biventricular repair is a surgical procedure that results in two separate ventricles pumping blood to the systemic and pulmonary circulations without any shunts. Univentricular repair refers to a surgical procedure in which the ventricle(s) are used exclusively for pumping blood to the systemic circulation, while the pulmonary blood flow is maintained with an aortopulmonary shunt, ventriculopulmonary shunt, or cavopulmonary shunt. Congenital heart diseases that require univentricular repair are called functionally single ventricle or univentricular heart diseases. They include double-inlet ventricles, tricuspid atresia, mitral atresia, hypoplastic left heart syndrome, critical aortic stenosis with hypoplastic left ventricle or severe endocardial fibroelastosis, and unbalanced atrioventricular septal defect. Additionally, hearts with two good-sized ventricles may be regarded as functionally single ventricular when the complex intracardiac anatomy precludes proper septation of the ventricles.
Biventricular versus univentricular repair. Cardiovascular surgical procedures can be divided into biventricular and univentricular repairs. A biventricular repair is a surgical procedure that results in two separate ventricles pumping blood to the systemic and pulmonary circulations without any shunts. Univentricular repair refers to a surgical procedure in which the ventricle(s) are used exclusively for pumping blood to the systemic circulation, while the pulmonary blood flow is maintained with an aortopulmonary shunt, ventriculopulmonary shunt, or cavopulmonary shunt. Congenital heart diseases that require univentricular repair are called functionally single ventricle or univentricular heart diseases. They include double-inlet ventricles, tricuspid atresia, mitral atresia, hypoplastic left heart syndrome, critical aortic stenosis with hypoplastic left ventricle or severe endocardial fibroelastosis, and unbalanced atrioventricular septal defect. Additionally, hearts with two good-sized ventricles may be regarded as functionally single ventricular when the complex intracardiac anatomy precludes proper septation of the ventricles.
 Glossary of Surgical Procedures
Glossary of Surgical Procedures
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree