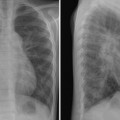
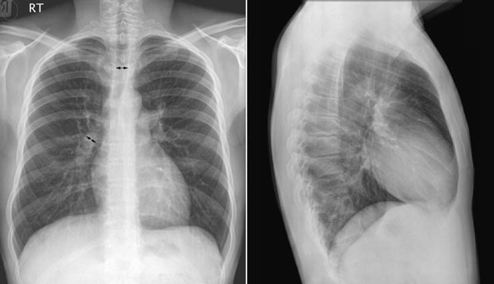
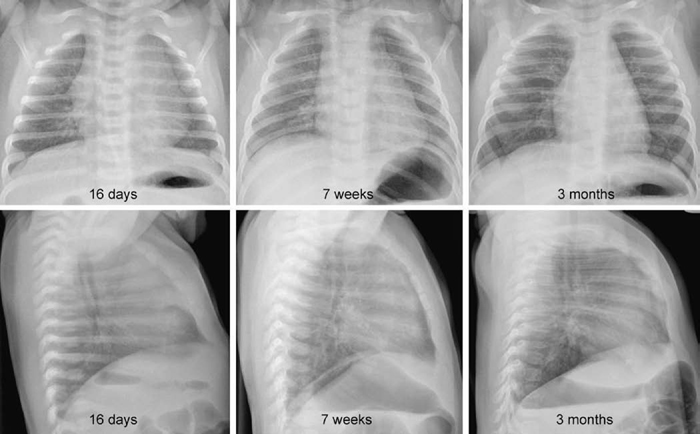
9 Pulmonary Vascularity The fourth step in chest radiographic interpretation is to assess the pulmonary vascular patterns. The pulmonary vascular patterns can be classified into six categories1: For example, this part of the report in a patient with unilateral absence of the right pulmonary artery can read as follows: “The pulmonary vascularity is asymmetric with the vascularity of the right lung markedly reduced and that of the left lung increased. The right lung hilum is not only overlain by the displaced heart but also inconspicuous. The right lung is diffusely reticular, which suggests systemic-to-pulmonary arterial collaterals. These findings in conjunction with a reduced volume of the right lung are highly suggestive of unilateral absence of the right pulmonary artery.” The normal pulmonary vascular markings show gradual tapering toward the periphery of the lungs (Fig. 9.1). Vascular markings are more prominent in the lower lungs than in the upper lungs, especially when the chest radiograph is obtained in an upright position. The apical parts of the lungs are devoid of sizable vascular markings in most normal individuals. Although the pulmonary vessels show some asymmetric anatomy in the lung hila, the pulmonary vascularity in the lungs is normally symmetric. The symmetry of the peripheral pulmonary vascularity should be assessed region by region. It is common to perceive the vascularity of the left lower lung as being slightly less prominent than that of the right lower lung. This is often a visual illusion. As a significant part of the left lower lung is overlain by the cardiac silhouette, the pulmonary vascularity of this area is perceived as less obvious than the vascularity of the opposite right lower lung. When the left hilum is uncovered on a chest radiograph obtained in a slight left anterior or right posterior oblique view, the vascularity of the left lung appears mistakenly increased. When the vascularity is symmetric, the descending branch of the right pulmonary artery can be used as a reference vessel for determination of pulmonary vascular prominence. Normally, the diameter of the descending branch of the right pulmonary artery is equal to the diameter of the trachea in the thorax (Fig. 9.1).2 In the peripheral lungs, the pulmonary vessels and adjacent bronchial lumina are equally sized. Fig. 9.1 Normal chest frontal and lateral views. The pulmonary vessels show gradual tapering toward the periphery. The descending branch of the right pulmonary artery has a diameter similar to that of the trachea in the thorax (double-headed arrows). RT, right. Fig. 9.2 Normal frontal and lateral radiographs in a series of young infants. Pulmonary vascularity appears diminished in the early neonatal period and becomes more prominent with age. A large thymus overlies the cardiac silhouette and central pulmonary vessels. The changing pattern of pulmonary vascularity is more obvious on the lateral images. Pulmonary vascularity appears less prominent in newborns and younger infants than in older children (Fig. 9.2). This is partly due to the small vessel size in normal newborns. It is also considered to be due to a difference in shape of the thoracic cage. Normally newborns and young infants have a deeper thoracic cage and therefore an increased ratio between the air-filled lung and soft tissue. Consequently, the lungs appear hyperlucent and the pulmonary vascularity less prominent. In addition, a large thymus overlies the larger vessels in the medial lung areas. Normal pulmonary vascularity can be seen in patients with a milder form of right- and left-sided lesions that is not complicated by an intracardiac or extracardiac shunt or heart failure (Table 9.1). Isolated right-sided lesions including pulmonary valve stenosis, pulmonary regurgitation, and tricuspid regurgitation usually show normal pulmonary vascularity. Decreased pulmonary vascularity in these lesions means that there is right-side heart failure or a right-to-left shunt through the patent foramen ovale. In isolated pulmonary valve stenosis, the pulmonary arterial segment of the left heart border shows a round bulge due to poststenotic dilatation (Fig. 9.3). The pulmonary vascularity within the lungs is usually normal. Occasionally the pulmonary vascularity may be mildly asymmetric because of a preferential pulmonary blood flow to the left lung. Isolated pulmonary regurgitation is also associated with normal peripheral pulmonary vascularity but with cardiomegaly and prominence of the main pulmonary arterial segment of the left heart border (Fig. 8.37, right upper panel). Mild to moderate forms of isolated tricuspid regurgitation are associated with normal pulmonary vascularity.
 Normal Vascularity
Normal Vascularity
| Right-sided lesions | Pulmonary stenosis with intact ventricular septum |
| Pulmonary regurgitation | |
| Mild tricuspid regurgitation | |
| Mild forms of left-sided lesions | Aortic valve stenosis |
| Supravalvar aortic stenosis | |
| Aortic regurgitation | |
| Coarctation of aorta |
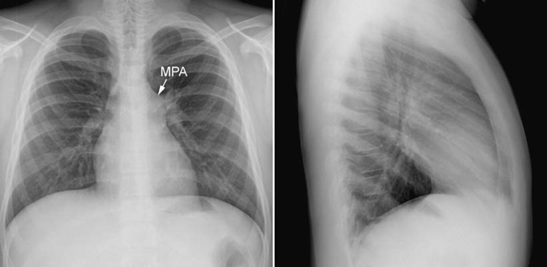
Fig. 9.3 Isolated pulmonary valvar stenosis. The main pulmonary arterial (MPA) segment of the left heart border shows a convex bulge due to poststenotic dilatation. Peripheral pulmonary vascularity is normal. A similar finding can also be seen as a normal variation.
Mild forms of left-sided lesions involving the aortic valve and aorta show normal pulmonary vascularity. However, mitral valve pathology is usually associated with a pulmonary venous hypertension pattern.
 Increased Vascularity (Plethora)
Increased Vascularity (Plethora)
Increased pulmonary vascularity is typically seen in acyanotic congenital heart diseases causing a left-to-right shunt (Table 9.2). It is also seen in cyanotic congenital heart diseases that are not associated with major obstruction to pulmonary arterial blood flow. In these conditions, interatrial or interventricular mixing of the systemic and pulmonary venous returns is necessary with the exchange in favor of the lower pressure pulmonary circulation, resulting in increased pulmonary vascularity. Finally, increased vascularity may also be due to high cardiac output status, which may be secondary to a large vascular malformation or hemangioma in the liver, brain, or extremities; severe anemia; and thyrotoxicosis.
Increased vascularity is characterized by uniformly enlarged vessels at the hila and within the lungs (Figs. 9.4, 9.5). The enlarged main pulmonary artery causes a convex bulge of the pulmonary arterial segment of the left heart border. The diameter of the descending branch of the right pulmonary artery exceeds the diameter of the trachea. The peripheral vessels within the lungs are bigger than the adjacent end-on shadows of bronchi. The peripheral pulmonary arteries can be tortuous. The margins of the enlarged vessels are sharp and clean unless there is associated pulmonary edema, atelectasis, or consolidation. Both increased and decreased vascularity can be perceived much more reliably on lateral radiographs than on frontal radiographs. Increased pulmonary vascularity is often not perceived when the pulmonary to systemic blood flow ratio (Qp/Qs) is under 2:1 (Fig. 9.6). Although the degree of increased pulmonary vascularity and heart size tend to correlate with the Qp/Qs, chest radiographs cannot be used as a quantitative measurement tool for the shunt lesions as both the pulmonary vascular prominence and heart size at a given amount of left-to-right shunt may vary according to the heart rate and contractility (Fig. 9.7). When the left-to-right shunt lesion is complicated by congestive heart failure, the margins of the prominent pulmonary vessels become indistinct (Fig. 9.8).
The magnitude of the left-to-right shunt through the atrial septal defect, ventricular septal defect, aortopulmonary window, and patent ductus arteriosus is dependent not only on the size of the defect but also on the differences in vascular resistance between the pulmonary and the systemic circulations.3 Low pulmonary vascular resistance causes a large shunt, whereas high pulmonary vascular resistance restricts the left-to-right shunt and may result in a reversed shunt. The pulmonary vascular resistance is high in fetal life because the small muscular pulmonary arteries have a thick muscular wall and small lumen.4 With initiation of pulmonary ventilation at birth, pulmonary vascular resistance declines rapidly. After the initial rapid decrease in pulmonary vascular resistance and pulmonary arterial pressure, there is a slow, progressive decrease, with adult levels reached after 2 to 6 weeks.5 This process can be delayed when there is a large shunt. Therefore, the left-to-right shunt lesions do not cause an overt increase in pulmonary vascularity and significant cardiomegaly in the first week of life. As the pulmonary vascular resistance gradually declines in the first few weeks or months of life, the pulmonary blood flow increases with larger amounts of shunt, and the cardiac chambers enlarge. If the lesion is left untreated, the lungs gradually develop obstructive vascular changes, which result in pulmonary hypertension (discussed later in this chapter).
| Lesions with left-to-right shunt | Partial anomalous pulmonary venous connection |
| Atrial septal defect | |
| Atrioventricular septal defect | |
| Ventricular septal defect | |
| Left ventricle-to-right atrial shunt | |
| Aorto-cameral fistula | |
| Coronary-cameral fistula | |
| Aortopulmonary window | |
| Patent ductus arteriosus | |
| Cyanotic heart diseases with bidirectional shunt | Fenestrated or unroofed coronary sinus |
| Common atrium | |
| Total anomalous pulmonary venous connection | |
| Double outlet right ventricle without pulmonary stenosis | |
| Double inlet ventricles without pulmonary stenosis or atresia | |
| Tricuspid atresia without pulmonary stenosis or atresia | |
| Complete transposition of the great arteries without pulmonary stenosis or atresia | |
| Pulmonary atresia with ventricular septal defect and unobstructed major aortopulmonary arterial collateral arteries | |
| Truncus arteriosus | |
| Hyperkinetic circulatory or high output states | Systemic arteriovenous malformation and hemangiomas, such as vein of Galen aneurysm, hepatic hemangioendothelioma and Klippel-Trenaunay-Weber syndrome |
| Severe anemia | |
| Thyrotoxicosis |
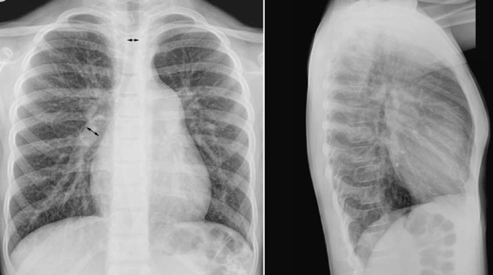
Fig. 9.4 Increased pulmonary vascularity in a 7-year-old patient with a secundum atrial septal defect. The diameter of the descending branch of the right pulmonary artery is greater than the diameter of the trachea (double-headed arrows). The main pulmonary arterial segment of the left heart border is convex. There was mild pulmonary hypertension with the systolic pulmonary arterial pressure of 28 mm Hg. The heart is moderately enlarged.
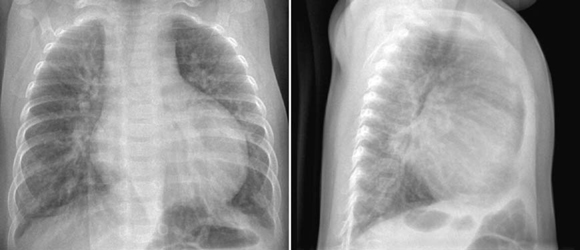
Fig. 9.5 Markedly increased pulmonary vascularity in a 5-month-old patient with a ventricular septal defect. The heart is markedly enlarged. Both lungs show air-trapping.
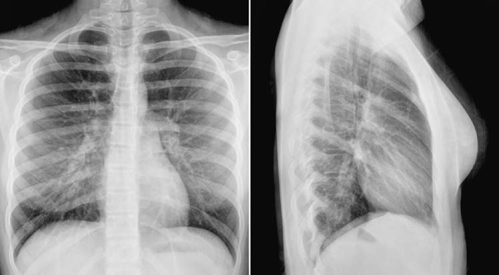
Fig. 9.6 Sinus venosus type atrial sepal defect with the right upper and middle pulmonary veins connecting to the superior vena cava in a 15-year-old girl. The ratio between the pulmonary and systemic blood flow (Qp/Qs) was 2.2 and right ventricular volume index was 151 mL/m2 of body surface area, which is moderately increased. The heart is normal in size and the pulmonary vascularity only mildly increased.
In certain conditions, the magnitude of the left-to-right shunt is relatively unaffected by the changes in pulmonary vascular resistance. In contrast to the conditions in which the magnitude of the shunt is dependent on pulmonary vascular resistance (dependent shunt lesions), these conditions are called obligatory shunt lesions.3 An obligatory shunt occurs when the shunt is between chambers having a large pressure difference, such as a left ventricle-to-right atrial shunt, which commonly occurs in an atrioventricular septal defect (Fig. 9.9). It also occurs when the shunt lesion is associated with a severe obstructive lesion downstream. For instance, a ventricular septal defect causes a larger amount of left-to-right shunt when it is associated with left ventricular outflow tract obstruction than when it is an isolated lesion (Fig. 9.10). Other obligatory shunt lesions include large arteriovenous malformations and aorto-right atrial or coronary artery to right atrial fistula. Increased pulmonary vascularity with overt cardiomegaly in the first few days of life is highly suggestive of an obligatory shunt lesion.
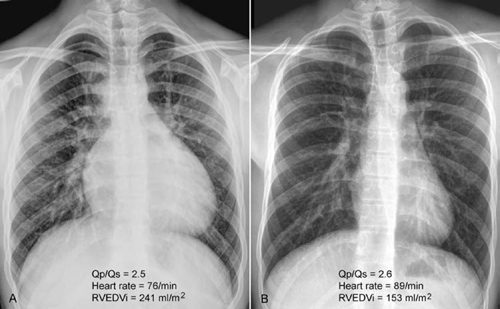
Fig. 9.7 (A,B) Frontal chest radiographs from two different 17-year-old patients with an atrial septal defect. The two patients had similar ratios between the pulmonary and systemic blood flow (Qp/Qs) but significantly different heart sizes and prominence of pulmonary vascularity. (B) Note that the patient with a normal heart size had a higher heart rate than the patient with a large heart (A). Pulmonary vascularity is moderately increased in A and mildly increased in B. RVEDVi, right ventricular end diastolic volume index.
As the bronchi are compressed by the accompanying dilated pulmonary arterial branches, generalized or multifocal obstructive emphysema or segmental or less commonly lobar collapse are often seen even without significant respiratory symptoms (Fig. 9.5).
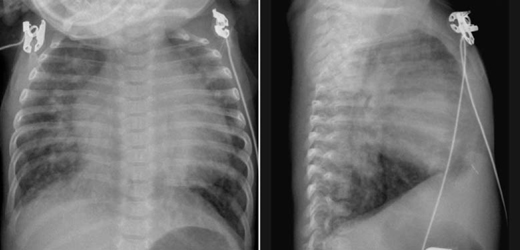
Fig. 9.8 Ventricular and atrial septal defects complicated by congestive heart failure in an 8-week-old infant. The pulmonary vascularity is markedly increased. The vessel margins are blurred because of interstitial edema. The heart is markedly enlarged. The lungs are hyperinflated with posterior atelectasis.
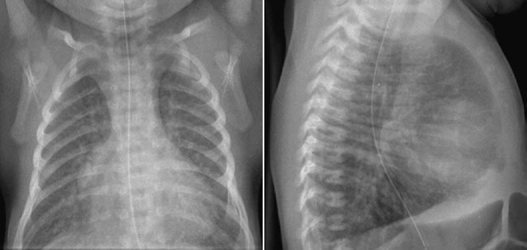
Fig. 9.9 Markedly increased pulmonary vascularity in an 11-day-old newborn with complete atrioventricular septal defect.
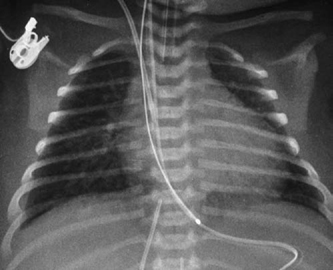
Fig. 9.10 Markedly increased pulmonary vascularity in a 4-day-old newborn with interrupted aortic arch and ventricular septal defect. There are signs of interstitial pulmonary edema and small bilateral effusions, suggesting congestive heart failure.
 Decreased Vascularity (Oligemia)
Decreased Vascularity (Oligemia)
Decreased vascularity is seen with a right-to-left shunt, right-sided heart failure, pericardial effusion or constrictive pericarditis, and pulmonary embolism (Table 9.3).
Decreased vascularity is characterized by uniformly small size of the pulmonary vessels at the hila and within the lungs (Fig. 9.11, 9.12, 9.13). As mentioned, determination of decreased as well as increased vascularity is often easier and perhaps more accurate on lateral radiographs than on frontal radiographs. As the main pulmonary artery and underlying ventricular outflow tract is also small, the pulmonary arterial segment of the heart border is less prominent than normal. The lungs may appear hyperlucent.
The heart size is normal or small when the right-to-left shunt is primarily associated with an obstructive lesion to the pulmonary blood flow but without valvular regurgitation as in tetralogy of Fallot and other complex congenital heart disease (Figs. 9.11, 9.12). However, tetralogy of Fallot may show normal or even increased vascularity and cardiomegaly when the pulmonary outflow tract obstruction is only mild in the initial few weeks to months of life (Fig. 9.14). The patient is typically acyanotic in this period and the condition is called “pink” tetralogy of Fallot. With time the right ventricle further hypertrophies due to increased pressure and volume overload. As the right ventricular outflow obstruction worsens with myocardial hypertrophy, right-to-left shunting eventually ensues, and the patient becomes cyanotic. Similar changes over time can also be seen in other cyanotic heart diseases with mild pulmonary outflow tract obstruction and a septal defect.
Decreased pulmonary vascularity is associated with cardiomegaly when there is severe valvular regurgitation or right-sided heart failure. Pulmonary atresia with intact ventricular septum is a good example showing both decreased pulmonary blood flow and an enlarged heart. Cardiomegaly in this condition is mainly due to tricuspid regurgitation causing severe right atrial dilatation, although left ventricular dilatation and right ventricular hypertrophy are additional contributing factors (Fig. 9.13).
Pulmonary vascularity is usually reduced when there is a moderate amount of pericardial effusion (Fig. 9.15A). In both cardiac tamponade and constrictive pericarditis, reduced pulmonary vascularity is associated with pulmonary venous hypertension (Fig. 9.15B).
| Lesions with right-to-left shunt | Tetralogy of Fallot |
| Pulmonary atresia with ventricular septal defect | |
| Pulmonary stenosis or atresia with intact ventricular septum, with right-to-left shunt at atrial level | |
| Ebstein’s malformation of tricuspid valve with right-to-left shunt at atrial level | |
| Complex congenital heart disease with pulmonary stenosis or atresia causing right-to-left shunt | |
| Right-sided heart failure | Right-sided heart failure |
| Uhl’s anomaly | |
| Pericardial disease | Pericardial effusion |
| Constrictive pericarditis | |
| Others | Widespread peripheral pulmonary artery stenosis |
| Pulmonary embolism | |
| Extrinsic compression of the pulmonary artery or right ventricular outflow tract due to tumor, aortic aneurysm, or mediastinal pathology |
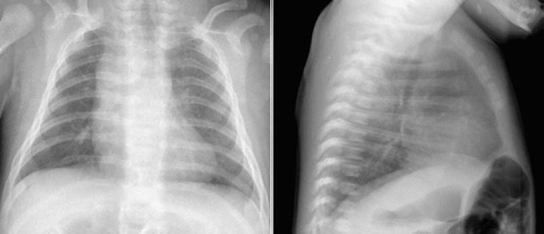
Fig. 9.11 Markedly decreased pulmonary vascularity in a newborn with a severe form of tetralogy of Fallot. The reduced vascularity is more obvious on the lateral view with better visualization of the small hilar pulmonary arteries. There is a right aortic arch.
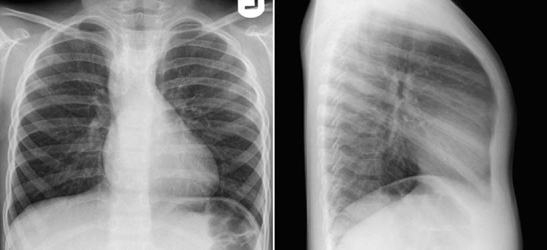
Fig. 9.12 Mildly decreased pulmonary vascularity in a 5-year-old patient with a mild form of tetralogy of Fallot. The pulmonary vascularity looks almost normal on the frontal view. The lateral view clearly shows that the vascularity is decreased as compared with normal vascularity shown in Fig. 9.1. There is a right aortic arch.
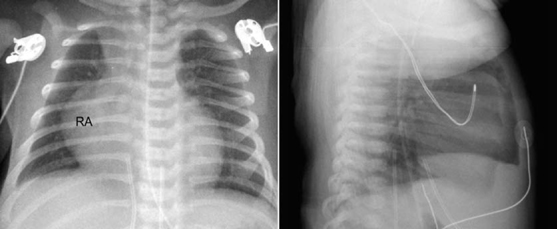
Fig. 9.13 Decreased pulmonary vascularity in a 4-day-old patient with pulmonary atresia with intact ventricular septum. There is moderate cardiomegaly with markedly enlarged right atrium (RA) due to severe tricuspid regurgitation.
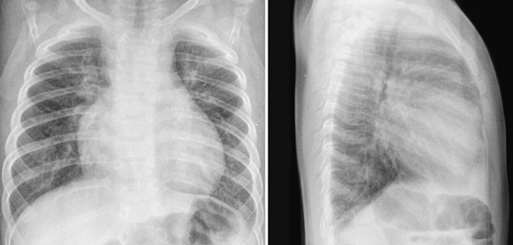
Fig. 9.14 Pink tetralogy of Fallot. Chest radiographs from a 5-month-old patient with mild form of tetralogy of Fallot show moderate cardiomegaly and increased pulmonary vascularity. The arterial oxygen saturation at room air was 98%.
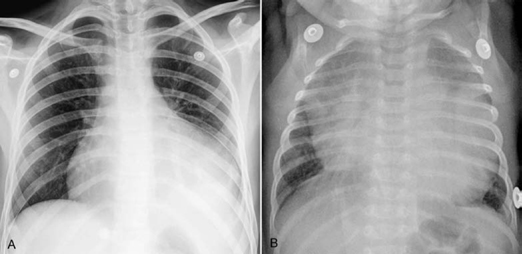
Fig. 9.15 Different manifestations of pericardial effusion. (A) This 15-year-old patient with underlying aortic stenosis was supposed to have a rather gradual accumulation of pericardial effusion. The enlarged cardiac silhouette shows a smooth contour. The pulmonary vascularity is reduced. The left lower lobe is collapsed. (B) This 11-week-old infant developed pericardial tamponade due to acute pericarditis. The cardiac silhouette is hugely enlarged and both lungs are edematous.
 Pulmonary Venous Hypertension
Pulmonary Venous Hypertension
Pulmonary venous hypertension is related to increased resistance to flow within the pulmonary veins. As the pulmonary venous pressure is not directly measurable, pulmonary capillary wedge pressure (PCWP) is used as an indirect measure of the pulmonary venous pressure. It is measured by inserting a balloon-tipped catheter into a peripheral vein. Once the catheter tip is advanced into a pulmonary arterial branch, the balloon is inflated and the pressure is measured. The normal mean PCWP is 6 to 12 mm Hg. Pulmonary venous hypertension is defined as a mean PCWP above 12 mm Hg.6 Pulmonary venous hypertension is caused by obstructive lesions of the pulmonary venous drainage route, mitral regurgitation, and left ventricular dysfunction (Table 9.4). The obstruction in the pathway of the pulmonary venous return may be caused by obstruction of the pulmonary veins, but can also be due to an obstructive lesion within the left atrium or left atrial outlet. Mitral regurgitation causes increased left atrial pressure that leads to pulmonary venous hypertension. Left ventricular dysfunction or failure causes elevation of the left ventricular diastolic pressure that leads to impaired diastolic flow into the left ventricle and thus to impaired pulmonary venous drainage (Table 9.4). Significant hypertrophy of the left ventricle from any etiology causes impaired relaxation or reduced compliance and therefore impaired filling of the left ventricle and ultimately results in pulmonary venous hypertension. Acute accumulation of pericardial effusion or constrictive pericarditis also may result in pulmonary venous hypertension because of impaired left ventricular filling.
| Obstruction to pulmonary venous drainage | Pulmonary veins | Obstructive types of total anomalous pulmonary venous connection |
| Pulmonary veno-occlusive disease (PVOD) | ||
| Individual pulmonary vein stenosis | ||
| Pulmonary vein atresia | ||
| Left atrium | Cor triatriatum | |
| Tumor (myxoma) | ||
| Thrombus |