This article is a review of vascular tumors and malformations that occur in infancy and childhood. It discusses anomalies of arterial, venous, capillary, lymphatic, and mixed vascular endothelium in terms of their varying forms, clinical course, imaging characteristics, complications, and treatment. The comparative utility of various imaging modalities is simplified.
Vascular anomalies of childhood, sometimes called birthmarks, are among the most common of all congenital and neonatal abnormalities. Many descriptive or histopathologic terms have previously been used to describe these anomalies (eg, strawberry hemangioma, port-wine stain, cavernous hemangioma). Although colorful, these terms were used inconsistently and often erroneously, potentially leading to inappropriate clinical management.
In a landmark publication in 1982, Mulliken and Glowacki proposed a system that now represents the international standard for classification of vascular anomalies in children. By examining the clinical and histopathologic features of vascular anomalies, these investigators sought to simplify categorization, thereby clarifying proper clinical management. After further refinement by multiple contributors, the modern classification scheme for vascular anomalies was accepted by the International Society for the Study of Vascular Anomalies (ISSVA) in 1992. Table 1 contains terms commonly used in the literature to describe vascular anomalies categorized based on that classification system.
| Category a | Colloquial Term |
|---|---|
| Hemangioma | Strawberry hemangioma Strawberry mark Mixed hemangioma Capillary hemangioma Capillary-cavernous hemangioma Juvenile hemangioma Cellular hemangioma |
| Vascular malformation | Port-wine stain (capillary) Port-wine hemangioma (capillary) Cavernous malformation (venous) Cavernous hemangioma (venous) |
a Based on the ISSVA-accepted classification approved in 1992. Note that the colloquial terms are inconsistently used in the literature. Consequently, the categorization in Table 1 is not applicable in all cases. For example, although the term port-wine stain has most frequently been used to describe capillary malformations, it has also been used to describe venous malformations in the past.
Vascular anomalies are now divided into 2 categories: vascular tumors, hemangiomas being by far the most common; and vascular malformations ( Table 2 ). The term hemangioma describes a lesion that undergoes a phase of proliferation involving high mitotic activity followed by a period of involution. In contrast, a vascular malformation shows normal endothelial turnover and growth commensurate with the child without spontaneous resolution. This category comprises malformations of arterial, venous, capillary, lymphatic, and mixed vascular endothelium. There are other vascular tumors (eg, Kaposiform hemangioendothelioma, tufted angioma, hemangiopericytoma) that are distinct from hemangiomas. These tumors are significantly less common and are not discussed in detail in this article.
| Category | Subcategory | Flow Characteristic |
|---|---|---|
| Tumor | Hemangioma of infancy | |
| Proliferative phase | High a | |
| Involutional phase | Low a | |
| RICH | High | |
| NICH | High | |
| Malformation | Arterial malformation | |
| Arteriovenous malformation | High | |
| Arteriovenous fistula | High | |
| Venous malformation | Low | |
| Capillary malformation | Low | |
| Lymphatic malformation | Low | |
| Mixed vascular malformations | Variable | |
a Just as the proliferative, quiescent, and involutional phases of hemangioma development exist as a continuum, so too do the respective flow velocities.
In most instances, accurate diagnosis of a hemangioma or vascular malformation requires consideration of both clinical findings and diagnostic imaging. Ultrasonography, magnetic resonance (MR) imaging, computed tomography (CT), and conventional angiography each have a role in assisting diagnosis, but ultrasound and MR imaging are optimal first-line diagnostic tools. These imaging modalities also play a central role in identifying appropriate treatment and assessing treatment efficacy.
Hemangiomas
Mulliken and Glowacki suggested that the term hemangioma be reserved for true neoplasms that arise during infancy, experience a proliferative phase, and eventually involute to some degree. Identifying this characteristic clinical course is vital to accurate diagnosis. The term hemangioma continues to be erroneously used to describe many birthmarks, vascular malformations or otherwise. Hemangiomas can be further divided into hemangiomas of infancy and congenital hemangiomas, which are less common and have generally completed their proliferative phase at the time of birth.
Although many hemangiomas can be diagnosed clinically, diagnostic imaging can assist in clarifying questionable diagnoses, excluding malignancies of similar appearance, identifying lesion depth and organ involvement for surgical planning, and in assessing treatment response. Histologically, the proliferative phase is characterized by high mitotic activity and the involutional phase by fibrosis and decreased cellularity. These cellular changes account for imaging findings that are specific to each evolutionary phase, particularly with MR imaging.
Hemangioma of Infancy
Also referred to as the infantile hemangioma or common infantile hemangioma, the hemangioma of infancy is the most common head and neck tumor of childhood. It is present in approximately 1% to 2% of all neonates and is approximately 5 times more common in girls than in boys. The incidence is reportedly even higher in white infants, 10% to 12% in full-term deliveries, and higher still in those born prematurely. Although most lesions (80%) are solitary, approximately 20% of affected infants present with more than 5 lesions.
The most commonly involved locations are the head and neck (60%), followed by the trunk (25%), and extremities (15%). Within the head and neck, lesions have been identified in the forehead ( Fig. 1 ), temple, cheek, orbital/periorbital region, lips, nasal cavity, larynx, within glands, and in the deep spaces of the neck.
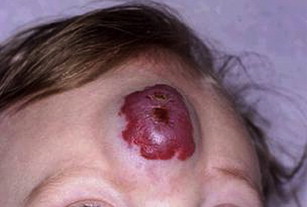
Most hemangiomas of infancy are not visible at birth, but present within the first several weeks of infancy. The proliferative phase, which begins in early infancy, involves rapid growth and can continue for 4 to 10 months. This is followed by a quiescent period and, later, an involutional phase of gradual regression in the next 6 to 10 years. Lesion appearance depends largely on depth, and 2 classic presentations have been described, although myriad forms have been reported. Superficial hemangioma (previously called strawberry hemangioma) describes a hemangioma of infancy that is superficial in location and generally bright red in color. In contrast, deep hemangioma (previously called mixed or cavernous hemangioma) describes a deeper lesion that may be colorless or have a bluish hue.
Complications can result from hemorrhage, ulceration, infection, or from a compromising lesion location. For example, orbital and periorbital hemangiomas can result in vision impairment or amblyopia. Airway involvement is common, particularly in those patients with hemangiomas of the chin and jaw line. Although most lesions ultimately leave no visible cosmetic defect, a minority can result in permanent pigmentation, scarring, or fibrofatty tissue.
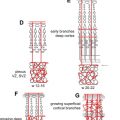
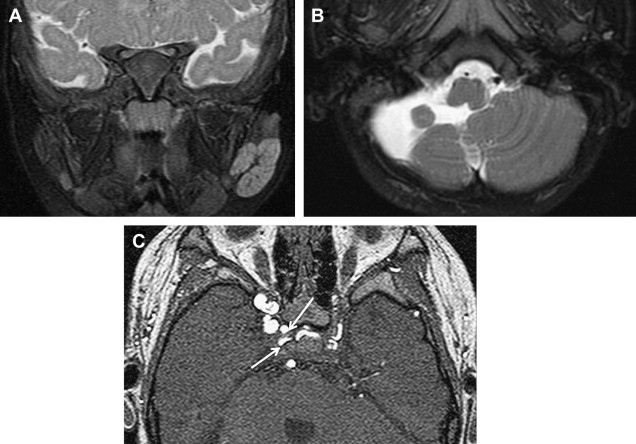
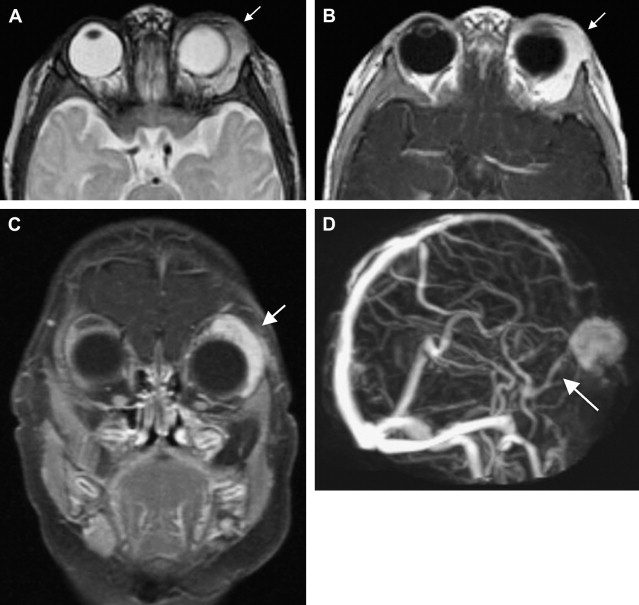
Several syndromes have been associated with hemangiomas, such as PHACES syndrome (posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and other cardiac defects, and eye abnormalities), and, traditionally, Kasabach-Merritt phenomenon. Characteristics of PHACES also include sternal clefts or supraumbilical raphes ( Fig. 2 ). Despite contradictions in the literature, Kasabach-Merritt (K-M) phenomenon (consumptive coagulopathy and thrombocytopenia) does not have an association with true hemangiomas. Current evidence suggests that the discolored, raised cutaneous lesions believed to be hemangiomas in K-M are kaposiform hemangioendotheliomas or tufted angiomas. The literature also contains associations with many other syndromes including Dandy-Walker, Klippel-Trenaunay, Sturge-Weber, Beckwith-Wiedemann, von Hippel-Lindau, and Osler-Weber-Rendu syndrome.
Some of these lesions involute spontaneously without cosmetic defect, and, in these cases, conservative watchful management is preferred. However, consideration must always be given to the anatomic area involved and the rate of growth of the lesion. The need for late surgical reconstruction can often be avoided by early intervention for hemangiomas located in critical areas such as the ear or nasal tip. Long-term functional sequelae from the temporary lesions must also be considered. For example, periocular hemangiomas frequently mandate treatment to prevent permanent vision impairment.
Treatment options include surgical excision, pharmacotherapy, chemotherapy, and laser therapy. Intralesional injection of corticosteroids is a common and effective means of promoting involution, particularly with small lesions. Oral corticosteroids have been shown to be effective in treating hemangiomas, with a 70% to 90% response rate. Systemic treatment is typically reserved for problematic lesions because potential side effects are numerous, including hypertension, fat deposition, mood alteration, and relative immunosuppression. However, these side effects can be easily minimized with the appropriate administration regimen. β-Blockers have recently been examined as a possible safe and effective alternative to corticosteroids. The risk/benefit ratio of this method, in comparison with corticosteroid therapy, remains to be established. In cases of life-threatening hemangiomas with failed prior therapy, vincristine and interferon-α have been used with high success rates. Laser therapy has also been used with variable success, particularly for thin hemangiomas causing skin irregularity and in treating areas of ulceration in larger hemangiomas. Intravenous steroids and surgical excision are the treatments of choice for life-threatening hemangiomas, those causing a loss of function, lesions unresponsive to less-invasive treatments, and for cases in which excessive redundant tissue is expected to persist after involution.
Congenital Hemangioma
The term congenital hemangioma refers to a hyperproliferative vascular lesion that completes its proliferative phase before birth. It has yet to be elucidated whether the congenital hemangioma is histopathologically distinct from, or simply a variant of, the hemangioma of infancy, but the natural history is distinct. Imaging characteristics of congenital and infantile hemangiomas are similar, but immunohistochemical analysis confirms that they are distinct entities. A specific glucose transporter protein, GLUT-1, has been identified that is specific to hemangiomas of infancy but expressed by neither congenital hemangiomas nor vascular malformations.
Two forms exist, the rapidly involuting congenital hemangioma (RICH) and the noninvoluting congenital hemangioma (NICH). Differentiating between congenital and infantile hemangiomas is done primarily with clinical information. RICHs complete involution more rapidly than hemangiomas of infancy, usually within the first 14 months of life. In contrast, NICHs do not involute but grow commensurately with the child. Because of the rapidity of regression, involuted RICHs can leave redundant skin, which can be cosmetically problematic. Differential diagnosis for the congenital hemangioma includes tufted angioma, embryonic rhabdomyosarcoma, fibrosarcoma, hemangiopericytoma, infantile myofibromatosis, and neuroblastoma.
Imaging characteristics
Although most hemangiomas can be diagnosed clinically, imaging can assist in confirming a clinical diagnosis, assessing tumor extent, and in surgical planning. Ultrasound and MR imaging are both reasonable first-line diagnostic tools for a suspected hemangioma. As a noninvasive, inexpensive, and increasingly available imaging modality, ultrasonography is ideal for evaluating a suspected hemangioma that is small in size and superficial in location. Particularly deep or large lesions may require MR imaging for complete characterization.
Congenital hemangiomas have the same imaging features as hemangiomas of infancy with a few exceptions. For example, congenital hemangiomas can have intravascular thrombi, which are not seen in the infantile form, larger venous components than infantile hemangiomas, and vascular aneurysms.
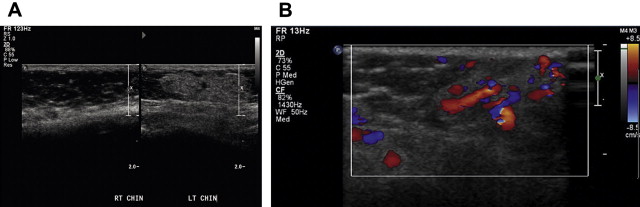
Grayscale ultrasonography depicts a well-demarcated structure of variable echogenicity ( Fig. 3 A). Color flow Doppler shows a highly vascular structure, which, in contrast with a vascular malformation, contains a sizeable parenchymal component (see Fig. 3 B). Hemangiomas are characterized by a high flow velocity during the proliferative phase and a lower flow velocity during the involutional phase. Unlike arteriovenous malformations, hemangiomas should not have an increased mean venous velocity.
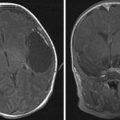
MR imaging is helpful in evaluating deep or large soft tissue masses. With hemangiomas of infancy, MR imaging shows a well-marginated soft tissue mass. T1-weighted sequences show a homogeneous lesion with intermediate signal intensity during the proliferative phase, and a heterogeneous lesion with small focal areas of fat replacement during the involutional phase ( Fig. 4 A). On T2-weighted images, lesions appear homogeneous and moderately hyperintense during the proliferative phase (see Fig. 4 B) and more heterogeneous while involuting. High-flow vessels, which are often at the lesion periphery, image as flow voids on spin echo sequences and as high signal intensity on gradient echo sequences. On contrast-enhanced T1-weighted sequences, hemangiomas enhance homogeneously (see Fig. 4 C). In the proliferative phase, MR angiography or venography may depict a large feeding artery or large draining veins ( Fig. 5 D). Phleboliths produce intralesional foci of low intensity, are uncharacteristic of hemangiomas, and should suggest an alternative diagnosis. Phleboliths are commonly seen in venous malformations and in mixed vascular malformations with a venous component.
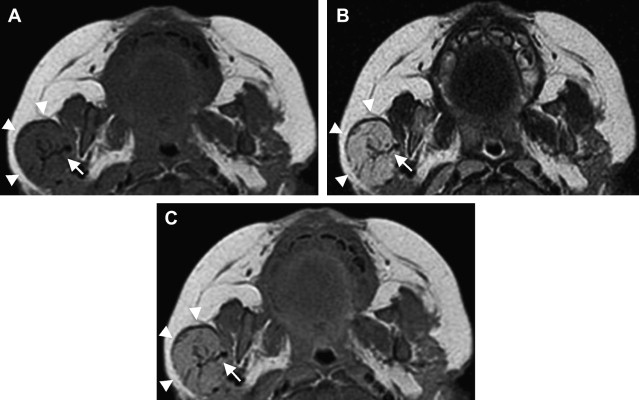
On nonenhanced CT, lesions appear homogeneous and isodense to muscle in the proliferative phase and heterogeneous with areas of low attenuation (fat) in the involutional phase. Contrast-enhanced CT images depict a lesion that uniformly enhances ( Fig. 6 ). Again, phleboliths are uncommon in true hemangiomas, and, if present, should suggest a vascular malformation with a venous component.
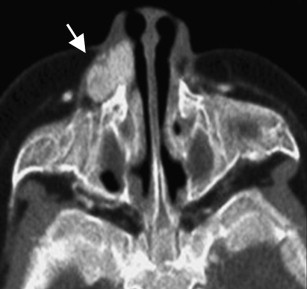
Conventional angiography shows a hypervascular mass with parenchymal enhancement. Enlarged feeding and draining vessels are commonly identified. Arteriovenous shunting has been documented but is uncommon.
Vascular malformations
Mulliken and Glowacki suggested that the term vascular malformation be used to describe anomalies of vasculature that are present at birth, grow proportionally with the child, have normal endothelial turnover, and do not spontaneously regress. Rather than a product of cellular hyperproliferation or neoplasia, these lesions result from abnormal vascular morphogenesis. This category includes arterial, venous, capillary, and lymphatic malformations, and any combination thereof.
Vascular malformations are categorized by primary constituent channel type (eg, arterial, venous, capillary) and subcategorized by relative flow velocity. These categories vary widely in potential complications and treatment challenges.
As with hemangiomas, the clinical presentation of vascular malformations depend on location and depth. By definition, these lesions are present at birth. However, they may not be visible or recognized until much later. Although the natural history of these lesions does not include a proliferative phase, they can grow in size. Hormonal changes, infection, trauma, and surgical injury can all result in enlargement. Unlike hemangiomas, vascular malformations can produce significant distortion of underlying bone.
Histologically, the vascular channels have normal cellularity and are lined with mature endothelium. Mitotic activity is not accelerated in these dysplastic structures, and they do not express biologic markers characteristic of vascular tumors, such as GLUT-1, FcγRII, merosin, and Lewis Y antigen.
Optimal treatment is dependent on subtype. Surgical resection, intralesional sclerotherapy, laser photocoagulation, chemotherapy, and pharmacotherapy have all been performed with varying degrees of success.
Venous Malformations
Venous malformations (VMs) are the most common of all vascular malformations. They are second only to hemangiomas as the most common vascular abnormality of the head and neck in childhood. The VM is frequently referred to as a cavernous hemangioma, although the entity is not a hemangioma.
These lesions, like all vascular malformations, are said to be present at birth, although they are frequently not identified until later in life. Composed of venous sinusoids of varying size, VMs have been described in intradermal, subcutaneous, intramuscular, and intraosseous locations. Common head and neck locations include the subcutaneous tissues of the face, muscles of mastication, periorbital region, and deep neck spaces. Lesion depth and location dictate clinical presentation. Superficial VMs are bluish in color, soft, and compressible ( Fig. 7 ). Some superficial lesions enlarge with Valsalva maneuver or with high-volume states. In contrast with arterial or arteriovenous malformations, these venous anomalies should not produce an auscultatory bruit or palpable thrill. Several syndromes are associated with VMs, including blue rubber bleb nevus, familial cutaneomucosal venous malformation, Klippel-Trenaunay, and Bockenheimer syndrome.
Conservative management, such as a compression device, is frequently preferred for uncomplicated lesions. Complications that indicate treatment include disfigurement, hemorrhage (including gastrointestinal bleeding), pain, and venous thrombosis. Traditionally, small focal lesions undergo complete surgical resection. Laser photocoagulation has also been used with reported success. At present, sclerotherapy is a commonly used first-line treatment of many low-flow anomalies including VMs. Sclerosants used in the treatment of VMs include ethanol, sodium tetradecyl sulfate, polidocanol, and ethanolamine oleate ( Fig. 8 B).
Imaging characteristics
When any vascular malformation is suspected, ultrasound is an ideal first-line diagnostic study, because the modality can characterize the lesion as low or high flow. MR imaging and CT are useful in defining lesion extent, involvement of vital organs, and identifying bone destruction. The low-flow characteristic of VMs accounts for many of their imaging features.
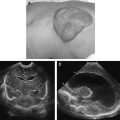
Grayscale ultrasound depicts the VM as a poorly marginated structure with vascular channels that are often compressible ( Fig. 9 ). The lesion is usually hypoechoic and heterogeneous and can appear infiltrative. Doppler spectral analysis shows monophasic low-velocity internal flow with no identifiable arterial waveform. Phleboliths, highly suggestive of a VM, image as echogenic foci with distal acoustic shadowing.
The use of intravenous gadolinium-based contrast is strongly recommended when low-flow lesions such as venous and lymphatic malformations are suspected. On T1-weighted imaging, these lobulated lesions appear hypointense to isointense compared with muscle ( Fig. 10 A). Heterogeneous signal may be perceived in the setting of intralesional hemorrhage or thrombosis. T2-weighted appearance varies with vascular channel size. Large channels produce a cystic and hyperintense appearance, secondary to the blood within, whereas smaller channels appear more solid with intermediate signal intensity (see Fig. 10 B). Venous thrombi produce low signal intensity on T2-weighted sequences. Unlike high-flow lesions, VMs generally do not produce flow voids on MR imaging. However, gradient echo sequences may show an absence of signal within lesion vessels, suggesting a low flow velocity. Phleboliths, highly suggestive of venous malformations, image as focal signal voids on T2-weighted studies. Contrast-enhanced MR imaging produces heterogeneous delayed enhancement (see Fig. 10 C).
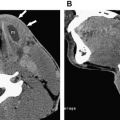
On CT, VMs appear as lobulated or multilobulated lesions, isodense to muscle, that can infiltrate multiple spaces of the head and neck. In contrast with hemangiomas, hyperdense rounded phleboliths may be present and are considered by some to be pathognomonic for the VM ( Fig. 11 ). In this respect, CT can prove a valuable modality in the diagnosis of VMs, because it readily identifies small calcified phleboliths. CT can also reveal bone remodeling adjacent to the lesion, a finding characteristic of VMs but uncommon to hemangiomas. Although they are benign lesions, the poor margination of VMs can give them an incongruously aggressive appearance. These low-flow lesions have small feeding arteries but may have enlarged draining veins. Contrast-enhanced CT usually shows enhancement, although to a variable extent because of the low-flow characteristic (see Fig. 8 A).