Hemophilia
In patients with severe or moderately severe hemophilia (decreased activity of coagulation factor VIII [hemophilia A] or IX [hemophilia B]), recurrent episodes of intraarticular bleeding during childhood and adolescence can produce mild to irreversible joint changes. The joints most commonly affected by hemophilic arthropathy are the knees, elbows, and ankles. The early initiation of prophylactic treatment with factor VII–IX concentrates has been recommended since the 1980s for the prevention of hemophilic arthropathy. Clinically, the severity of the disease is typically determined by the degree of ankle joint involvement.
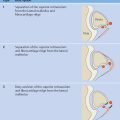
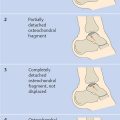
Two main types of scoring system are used in the radiologic evaluation of hemophilic arthropathy: progressive scores (e.g., the Arnold–Hilgartner score), in which the finding with the highest grade determines the final score, and additive scores (e.g., the Pettersson score), in which each finding is scored separately and the values are added together to yield a final score.
Pettersson Score
Pettersson et al published a score in 1980 that has become the most widely established system for evaluating hemophilic arthropathy on conventional radiographs. The Pettersson score has also been recommended for follow-ups by the World Federation of Hemophilia since 1981 and is applicable to all large joints.
The Pettersson score ( Table 14.1 ) covers eight parameters that are scored on radiographs of the affected joint taken in two planes. The extent of irreversible joint changes is assessed by scoring each of the parameters on a 0–2-point scale. Essentially reversible findings such as soft-tissue swelling or synovial thickening are disregarded. The maximum possible score for a given joint is 13 points. The score describes the natural progression of hemophilic arthropathy and has proven more accurate than other classifications (e.g., the Arnold–Hilgartner score) in discriminating among the various stages of the disease.
Because synovial and cartilaginous changes precede irreversible bone changes, the Pettersson score tends to underestimate the severity of arthropathy in the early stages of the disease (total score: 0–4).
Magnetic Resonance Imaging Scores
Since the 1990s, magnetic resonance imaging (MRI) has been used increasingly to detect synovial proliferation, incipient cartilage damage, and hemosiderin deposition in patients with hemophilic arthropathy. Although numerous scores have been proposed for this imaging modality, most are still undergoing clinical validation and no single score has yet been established as a standard method.
Denver Score
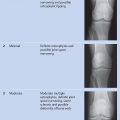
The Denver score ( Table 14.2 ) is a simple scoring system for everyday clinical practice and has already been used successfully in large clinical therapeutic trials. It is a progressive system in which the final score equals the highest grade that is assigned to any finding. It includes the assessment of effusion, hemarthrosis, synovial hypertrophy, hemosiderin deposits, subchondral cysts, and erosive changes. Articular cartilage loss is considered the end stage and receives the highest score.
Since the Denver score was introduced, the authors of that score as well as other authors have described several modifications and expansions, although none are used as frequently as the original score. Additive scales like that developed by Mathew et al (2000) are also used at selected centers but yield a cumulative score based on the sum of individual changes.
Funk MB, Schmidt H, Becker S, et al. Modified magnetic resonance imaging score compared with orthopaedic and radiological scores for the evaluation of haemophilic arthropathy. Haemophilia 2002;8(2):98–103. Lundin B, Pettersson H, Ljung R. A new magnetic resonance imaging scoring method for assessment of haemophilic arthropathy. Haemophilia 2004;10(4):383–389. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007;357(6):535–544. Mathew P, Talbut DC, Frogameni A, et al. Isotopic synovectomy with P-32 in paediatric patients with haemophilia. Haemophilia 2000;6(5):547–555.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree