The hepatic storage disorders are genetic conditions characterized by the accumulation of toxic substances within either hepatocytes or the hepatic extracellular matrix. This deposition causes secondary tissue damage, which may eventually progress to cirrhosis, portal hypertension, and hepatocellular carcinoma (HCC). As genetic conditions, their manifestations are wide-ranging and systemic, with hepatic involvement only one component of the larger illness. The most common of these disorders, hereditary hemochromatosis, is discussed in detail in its own chapter on hepatic iron overload. In this chapter, the focus is on the other relatively common storage disorders with hepatic manifestations: Wilson’s disease, alpha-1 antitrypsin (A1AT) deficiency, and the glycogen storage diseases (GSDs). Other storage disorders, such as the porphyrias, amyloidosis, and lysosomal storage diseases (Gaucher’s and Niemann-Pick diseases), are either very rare or primarily affect extrahepatic tissues. Nonalcoholic fatty liver disease shares some features of the storage disorders but is not inherited in mendelian fashion and so is not included in this disease category.
Etiology
Wilson’s disease, A1AT deficiency, and the GSDs are familial conditions inherited in autosomal recessive fashion caused by mutations in putative genes.
Wilson’s disease, also called hepatolenticular degeneration, is a disorder of copper metabolism. It is characterized by progressive neurologic deterioration and chronic liver disease leading to cirrhosis. The gene responsible for this disease is ATP7B, located on chromosome 13. Highly expressed in the liver, kidney, and placenta, it encodes a metal-transporting, copper-dependent P-type adenosine triphosphatase that functions in the incorporation of copper into ceruloplasmin (plasma protein that binds copper) and excretion of excess copper into bile.
A1AT deficiency is associated with the development of pulmonary emphysema, chronic liver disease, and HCC. It is caused by mutations in the SERPINA1 (formerly known as PI ) gene located on chromosome 14, which encodes the A1AT serine protease.
The GSDs are a heterogeneous group of inborn errors of metabolism characterized by excessive glycogen content of the liver and muscles (among other tissues, including the kidneys and spleen) as a result of enzyme defects in glycogen synthesis or degradation. Enzymatic deficiencies in nearly every step of glycogen processing have been identified, accounting for at least 10 discrete diseases that are grouped into the GSDs (types 0, I, II, III, IV, V, VI, VII, IX, and XI). This chapter focuses on type I GSD (von Gierke’s disease), by far the most common GSD associated with hepatic involvement. The other three GSDs associated with liver disease, types 0, III (Cori’s or Fanconi’s disease), and IV (Andersen’s disease), are rare.
Type I GSD is caused by mutations in the G6PC gene. Located on chromosome 13, G6PC encodes glucose-6-phosphatase, a vital enzyme of glycogenolysis. Individuals heterozygous for the G6PC mutation have no phenotypic expression.
Pathogenesis
The pathogeneses of Wilson’s disease, A1AT deficiency, and the GSDs are explained by the metabolic defects caused by their underlying genetic abnormalities.
Wilson’s Disease
The liver is the main organ responsible for copper homeostasis. Normal copper metabolism begins with the absorption of dietary copper by duodenal enterocytes and its transportation to hepatocytes via the portal circulation. Hepatocytes then excrete the copper into bile, leading to biliary copper excretion, which eventually results in fecal copper loss.
Genetic defects in the ATP7B protein are associated with diminished incorporation of copper into ceruloplasmin and reduced excretion of copper into the bile. Unincorporated copper accumulates within hepatocytes, where it causes secondary oxidative tissue damage. Some of the excess copper enters the systemic circulation and is deposited in extrahepatic sites such as the brain (especially the basal ganglia and limbic system), cornea, and kidneys. Copper not deposited in tissues is excreted in the urine. Ceruloplasmin not incorporated with copper is released into the bloodstream and rapidly degraded.
Alpha-1 Antitrypsin Deficiency
A1AT is normally synthesized in the liver and released into the blood. An acute-phase reactant, it is elevated during inflammation, infection, and cancer. Its most important physiologic role is to inactivate proteolytic enzymes in the lung (especially neutrophil elastase), which degrade lung matrix tissue after being released as a by-product of cellular immune responses to airborne pathogens. A1AT counterbalances this proteolytic activity, preventing the net degradation of the lung matrix and alveoli.
In A1AT deficiency, hepatic production of A1AT is compromised and pulmonary proteolytic activity is unopposed, resulting in chronic obstructive pulmonary disease (COPD) and emphysema. Liver disease is uncommon except in some forms of A1AT deficiency, which leads to a cascade of cellular events, including autophagy, mitochondrial injury, caspase inactivation, and hepatocellular damage. Eventually, fibrosis and cirrhosis may ensue.
Glycogen Storage Diseases
Glycogen is a highly-branched glucose polysaccharide. It is found in greatest concentration in the liver, and it functions as the body’s primary form of short-term energy storage during fasting periods. In the GSDs, enzyme defects in glycogen metabolism lead to accumulation of glycogen or glycogen metabolites, resulting in hepatocyte swelling, marked hepatomegaly, and hypoglycemia.
In type I GSD, a deficiency in glucose-6-phosphatase (G6P) results in glycogen accumulation within hepatocytes and causes hepatocellular damage via oxidative reactions. The damaged hepatocytes form neoplasms (hepatic adenomas and HCCs) with relatively high frequency. Despite the hepatocellular damage and steatosis associated with type I GSD, liver fibrosis and cirrhosis do not occur.
The other GSDs associated with liver disease (types 0, III, and IV) are caused by enzymatic defects at other steps in glycogen metabolism; these disorders are associated with progressive liver disease, leading to cirrhosis and portal hypertension.
Pathogenesis
The pathogeneses of Wilson’s disease, A1AT deficiency, and the GSDs are explained by the metabolic defects caused by their underlying genetic abnormalities.
Wilson’s Disease
The liver is the main organ responsible for copper homeostasis. Normal copper metabolism begins with the absorption of dietary copper by duodenal enterocytes and its transportation to hepatocytes via the portal circulation. Hepatocytes then excrete the copper into bile, leading to biliary copper excretion, which eventually results in fecal copper loss.
Genetic defects in the ATP7B protein are associated with diminished incorporation of copper into ceruloplasmin and reduced excretion of copper into the bile. Unincorporated copper accumulates within hepatocytes, where it causes secondary oxidative tissue damage. Some of the excess copper enters the systemic circulation and is deposited in extrahepatic sites such as the brain (especially the basal ganglia and limbic system), cornea, and kidneys. Copper not deposited in tissues is excreted in the urine. Ceruloplasmin not incorporated with copper is released into the bloodstream and rapidly degraded.
Alpha-1 Antitrypsin Deficiency
A1AT is normally synthesized in the liver and released into the blood. An acute-phase reactant, it is elevated during inflammation, infection, and cancer. Its most important physiologic role is to inactivate proteolytic enzymes in the lung (especially neutrophil elastase), which degrade lung matrix tissue after being released as a by-product of cellular immune responses to airborne pathogens. A1AT counterbalances this proteolytic activity, preventing the net degradation of the lung matrix and alveoli.
In A1AT deficiency, hepatic production of A1AT is compromised and pulmonary proteolytic activity is unopposed, resulting in chronic obstructive pulmonary disease (COPD) and emphysema. Liver disease is uncommon except in some forms of A1AT deficiency, which leads to a cascade of cellular events, including autophagy, mitochondrial injury, caspase inactivation, and hepatocellular damage. Eventually, fibrosis and cirrhosis may ensue.
Glycogen Storage Diseases
Glycogen is a highly-branched glucose polysaccharide. It is found in greatest concentration in the liver, and it functions as the body’s primary form of short-term energy storage during fasting periods. In the GSDs, enzyme defects in glycogen metabolism lead to accumulation of glycogen or glycogen metabolites, resulting in hepatocyte swelling, marked hepatomegaly, and hypoglycemia.
In type I GSD, a deficiency in glucose-6-phosphatase (G6P) results in glycogen accumulation within hepatocytes and causes hepatocellular damage via oxidative reactions. The damaged hepatocytes form neoplasms (hepatic adenomas and HCCs) with relatively high frequency. Despite the hepatocellular damage and steatosis associated with type I GSD, liver fibrosis and cirrhosis do not occur.
The other GSDs associated with liver disease (types 0, III, and IV) are caused by enzymatic defects at other steps in glycogen metabolism; these disorders are associated with progressive liver disease, leading to cirrhosis and portal hypertension.
Prevalence and Epidemiology
Wilson’s disease is present across almost all races and ethnicities, with a roughly even male-to-female distribution. The prevalence is 1 per 30,000 persons, and the carrier frequency is 1 in 90. Clinical presentation is usually in the second or third decade of life, although it has been described in patients younger than 5 years of age and rarely older than age 45.
The incidence of A1AT deficiency is approximately 1 in 2000 live births. Men and women are affected equally. In children, A1AT is the most common genetic cause of liver disease. The mean life span is 65 years in nonsmokers and is reduced to 50 years in smokers.
As a class, the GSDs occur in approximately 1 in 25,000 births; type I GSD has a prevalence of approximately 1 in 100,000 to 200,000 births. Nearly all cases have been identified in individuals from North America, Europe, or the Middle East. There is no predilection for ethnicity, race, or gender. Seventy percent of patients are diagnosed before the age of 2 years, with the development of hepatic adenomas in the second decade of life.
Clinical Presentation
Wilson’s Disease
Wilson’s disease manifests over a wide spectrum and may involve hepatic or neuropsychiatric sequelae, either together or alone. The presentation may be either acute or chronic; acute disease typically manifests as fulminant hepatic failure, whereas chronic disease consists of chronic hepatitis, cirrhosis, and neuropsychiatric illness. Patients who present with neuropsychiatric symptoms typically have asymptomatic hepatic involvement and are generally older than those who present with hepatic disease. Those presenting with hepatic disease typically will develop neuropsychiatric symptoms within 2 to 5 years.
Hepatic involvement occurs as either fulminant hepatic failure or a progressive, indolent chronic hepatitis that may ultimately result in cirrhosis. In the fulminant form, patients undergo rapid hepatic deterioration, with coagulopathy, encephalopathy, and renal failure. The chronic form develops over a period of decades, ultimately leading to the typical findings of cirrhosis and complications of portal hypertension. HCC is a rare complication, with fewer than 20 documented cases, but would predictably occur in those with long-standing Wilson’s disease. Neuropsychiatric symptoms are the initial manifestation in 40% to 50% of patients.
No single test determines the diagnosis of Wilson’s disease, although an amalgamation of clinical and biochemical findings, as well as pedigree analysis, is suggestive in the correct clinical scenario. Serum aminotransferase levels are typically mildly elevated (<200 international units/L), with a proportional increase in total bilirubin (<4.0 mg/dL). Interestingly, the serum alkaline phosphatase concentration is reduced in fulminant disease; thus, a ratio of alkaline phosphatase to total bilirubin of less than 2 is highly suggestive of Wilson’s disease in patients with fulminant hepatic failure. Copper-specific findings include elevated serum copper and 24-hour urinary copper levels, as well as decreased serum ceruloplasmin levels. In some cases, liver biopsy is indicated with attention placed on the hepatic copper content.
Alpha-1 Antitrypsin Deficiency
Pulmonary disease is usually more severe than hepatic disease and may occur in isolation; hepatic disease in the absence of pulmonary disease is rare. Pulmonary disease is accelerated by noxious stimuli, such as tobacco and air pollutants. Generally manifesting in early adulthood, it eventually progresses to severe panacinar emphysema (predominantly in the lower lobes) and COPD, characterized by bronchial hyperreactivity. Recurrent pulmonary infections are common.
Hepatic manifestations may occur in neonates as isolated disease (e.g., without concurrent pulmonary disease) or in adults with pulmonary disease. The neonatal manifestation is that of hepatitis with cholestasis, resulting in hepatomegaly and jaundice 4 to 8 weeks after birth, which spontaneously resolves after a few weeks. The presence of neonatal disease does not predict hepatic disease in adulthood. In adults, hepatic disease manifests as hepatitis, eventually progressing to fibrosis and cirrhosis. Cirrhosis develops slowly, typically requiring 20 to 30 years for portal hypertension to occur, and is the most common stage of presentation. Patients may exhibit complications of portal hypertension, including variceal bleeding, hypersplenism, ascites, and hepatic encephalopathy. The incidence of HCC is thought to be higher in patients with A1AT deficiency, although its transformation rate has not been well characterized. The diagnosis of A1AT deficiency should be considered in (1) any young patient with obstructive lung disease or in any person with concurrent lung and liver abnormalities; (2) a patient presenting with hepatomegaly, elevated transaminase or bilirubin levels, signs of portal hypertension, or cholestasis; (3) an individual with chronic hepatitis or cirrhosis of unknown cause.
Glycogen Storage Diseases
Type I GSD clinically manifests in the neonate, usually becoming evident within the first week of life. The chief systemic metabolic abnormality is hypoglycemia, typically in the range of 25 to 50 mg/dL. G6P accumulation in the kidneys causes nephromegaly and may result in proteinuria, systemic hypertension, or Fanconi’s syndrome. G6P accumulation in the liver causes hepatomegaly. By the second decade of life, hepatic adenomas develop in up to 75% of patients with type I GSD; these adenomas are considered premalignant and may transform into HCC in both pediatric and adult patients.
Pathology
Wilson’s Disease
The hepatic manifestations of excess copper accumulation are variable and often progressive. In early disease, patients demonstrate nonspecific signs of injury. In fulminant disease, massive hepatic necrosis is seen. In some patients, chronic inflammation may be present with increased numbers of lymphocytes in portal tracts, which can mimic chronic viral hepatitis. In advanced disease, progressive fibrosis leads to portal-portal bridging with eventual macronodular cirrhosis. Histochemical staining with rhodanine may be used to detect cytoplasmic copper; however, staining may be focal and missed on needle biopsy. Electron microscopy reveals characteristic mitochondrial abnormalities, including swollen cristae and crystalline inclusions.
Alpha-1 Antitrypsin Deficiency
Accumulated hepatocellular A1AT can be detected by histochemical staining with diastase-positive periodic acid–Schiff (PAS-D) or by immunohistochemical staining for A1AT. PAS-D staining reveals characteristic inclusions consisting of round-to-oval, purple-red globules within the cytoplasm of periportal hepatocytes. They may display hepatitis with features of canalicular cholestasis, giant cell transformation, swollen (“ballooning”) hepatocytes, and diminished bile ducts. Other histologic findings in the liver are nonspecific and include fatty change and, rarely, Mallory hyaline.
Glycogen Storage Diseases
In type I GSD, accumulation of glycogen metabolites within hepatocytes leads to hepatic enlargement. These metabolites may be seen on light microscopy as intracellular cytoplasmic vacuoles. The hepatocytes take on a pale appearance with prominent cell membranes.
Imaging
The diagnosis of Wilson’s disease is generally based on clinical and laboratory findings; complicated cases are confirmed by increased hepatic copper concentration in biopsy samples of liver tissue. In patients with cirrhosis secondary to Wilson’s disease, abdominal imaging may play a role in surveillance for HCC and detecting complications of portal hypertension.
A1AT deficiency primarily affects the lungs. Pulmonary manifestations include panlobular emphysema, predominantly involving the lung bases. Liver abnormalities are less frequent and usually less severe. There are no characteristic liver imaging findings. As with Wilson’s disease, abdominal imaging is used in advanced disease for the assessment of stigmata of cirrhosis and portal hypertension, as well as surveillance for HCC.
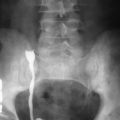
Accumulation of glycogen metabolites within the liver, kidneys, and spleen may result in enlarged organs, manifesting as hepatomegaly ( Figure 40-1 ), bilateral nephromegaly ( Figure 40-2 ), and splenomegaly, respectively. The liver is the most frequently involved organ, and hepatomegaly may be massive, fully occupying the abdomen and extending into the pelvis. A variable degree of fat accumulation within the liver parenchyma may also be evident. Because of the high incidence of adenomas and the potential risk for malignant transformation, patients with type I GSD usually undergo periodic imaging studies to assess for and monitor hepatic tumors.
Computed Tomography, Magnetic Resonance Imaging, and Ultrasound
Wilson’s Disease
In the early stages of Wilson’s disease, the liver usually has a normal imaging appearance, although nonspecific findings such as hepatomegaly may be observed. On unenhanced CT images, the liver may have increased attenuation as a result of deposited copper. However, there is no correlation between the degree of CT attenuation and hepatic copper concentration and the CT findings do not permit quantitative assessment of copper deposition. Copper has no ferromagnetic effect on MRI, and the signal intensity of the involved liver is usually normal. Similarly, copper does not scatter the ultrasound beam and the affected liver has normal echogenicity.
In fulminant presentations, necrotic portions of the liver may fail to enhance after intravenous administration of contrast agents. In advanced chronic cases, a macronodular contour of cirrhosis is apparent. This is indistinguishable from that of other causes of end-stage liver disease, although Wilson’s disease can be accompanied by normal to decreased caudate lobe size, and high hepatic attenuation on unenhanced CT due resulting from deposition ( Figure 40-3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree