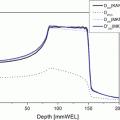
Fig. 25.1
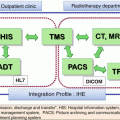
A patient with hepatocellular carcinoma 5.5 cm in maximum diameter in the right posterior interior segment of the liver. (a) CT scan before the treatment. (b) CT image for treatment planning: the arrow shows a metal marker implanted near the tumor. (c) X-ray image. The metal marker can be seen (arrow)
25.4 Treatment Planning
Three-dimensional treatment planning with 2.5-mm CT images was performed using the HIPLAN, which was originally developed for 3D treatment planning [17]. The planning target volume (PTV) was defined so as to include a 1 to 1.2-cm margin around the gross tumor volume. Dose-volume histogram was used to evaluate the probability of liver damage (Fig. 25.2). Double right-angled field geometry was used for irradiation in most patients. Respiratory gating was employed for CT image acquisition and irradiation stages to ensure more accurate delivery of the radiation [18]. To assess the accuracy of patient positioning and target volume localization, orthogonal fluoroscopy and radiography were used immediately prior to each treatment session.
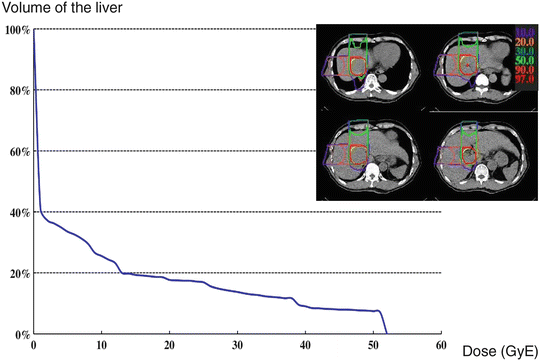

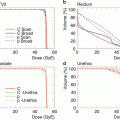
Fig. 25.2
Dose distribution and dose-volume histogram of the liver. In the treatment planning, a dose-volume histogram is used to evaluate the probability of liver damage
In order to prevent/minimize adverse events in certain organs, special attention is required in the treatment planning. To reduce rib fracture, the PTV margin should be defined so as not to include the outside of ribs in the high-dose area. Because the tumor usually does not exist on the outside of the liver surface, the PTV margin outside the liver can be set at less than that in the liver (Fig. 25.3). Skin should not be included in the area of a dose exceeding 70 % of the prescribed dose to avoid severe skin reaction. Great caution is also needed for setting the PTV margin so as not to irradiate the digestive tracts with high doses. If the gut exists near the tumor, the treatment beams should be set not to pass through the gut, if possible. If digestive tracts almost touch the tumor, high-dose irradiation is difficult because of the risk of serious gastrointestinal tract damage. In addition, the major trunk or main branch of the portal vein should not be fully included in the PTV to avoid liver failure and especially in cirrhotic liver.
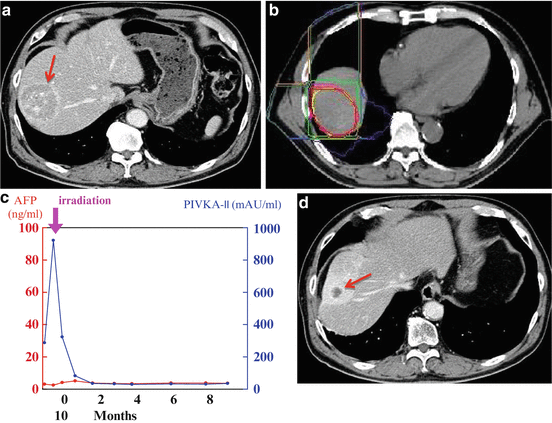

Fig. 25.3
A case with hepatocellular carcinoma 5.5 cm in maximum diameter in the right anterior superior segment of the liver. (a) CT scan before treatment. (b) Dose distribution: The beam was delivered in combination with anterior and lateral portals (dose ratio 1:1). The planning target volume was defined so as to include a 1.2-cm margin around the gross tumor volume except the right side where the margin was narrower to exclude the ribs from the high-dose area. (c) Change of serum tumor markers: The serum PIKA-II value promptly decreased after the treatment. (d) CT scan 9 months after treatment; the tumor had regressed remarkably. There were no rib fractures after the treatment
25.5 Short-Course Carbon-Ion Radiotherapy
Between April 2003 and August 2012, 133 patients with HCC underwent 2-fraction C-ion RT. Background data of the patients and tumors are presented in Table 25.1. The patients consisted of 91 males and 42 females. Median age was 72 years (range, 44–87). Median maximum tumor diameter was 42 mm (range, 14–140). One hundred and twenty-three patients were classified into Child-Pugh A and ten into Child-Pugh B. All 133 patients were treated with a total dose range of 32.0–45.0 GyE.
Table 25.1
Patient and tumor characteristics in 2 fractions of C-ion RT
Characteristics | Total (n = 133) |
|---|---|
Age (years) | |
Median | 72 |
Range | 44–87 |
Sex, n (%) | |
Male | 91 (68) |
Female | 42 (32) |
Etiology of cirrhosis, n (%) | |
HCVAb positive | 92 (69) |
HBsAg positive | 12 (9) |
None | 30 (23) |
Both | 1 (1) |
Child-Pugh classification, n (%) | |
A | 123 (92) |
B | 10 (8) |
UICC 5th stage, n (%) | |
I | 19 (14) |
II | 97 (73) |
IIIA | 15 (11) |
IVA | 2 (2) |
Number of tumors, n (%) | |
Single | 118 (89) |
Multiple | 15 (11) |
Maximum tumor diameter (mm) | |
Median | 42 |
Range | 14–140 |
AFP (ng/mL) | |
Mean ± SD | 2,350.3 ± 11,891.4 |
PIVKA-2 (mAU/mL) | |
Mean ± SD | 5,256.3 ± 43,838.0 |
No treatment-related deaths occurred in the hypofractionation trial. There were no cases of grade 4 hepatic toxicity. With regard to the Child-Pugh score, in the late phase (3 months after treatment), an increase of two points or more occurred in 6 and 3 % in the smaller-tumor group (≦5 cm) and the larger-tumor group (>5 cm), respectively. There was no significant difference between the two groups (P = 0.678). This demonstrated that the changes in liver function remained minor after C-ion RT was performed. No serious adverse effects were noted in the digestive organs. There was no other grade 4 or higher toxicity (Table 25.2).
Table 25.2
Acute and late toxicities after C-ion RT
Acute (n = 133) | Late (n = 132) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Grade | Grade | |||||||||
0 |
||||||||||