Ablatherm integrated imaging
Sonablate 500
Company
EDAP TMS SA, France
SonaCare Inc., USA
Since
2005
2001
Approval
Table
Integrated
Standard
Patient position
Right lateral
Lithotomy or supine
Frequency
7.5 MHz for treatment planning; 3 MHz for treatment
4 MHz for treatment and planning
Focal point
4.5 cm
3–5 cm
Transducer
Single-treatment probe with two transducers
Single-treatment probe with two transducers of different focal lengths
Power
Predefined for each treatment
Manual adjustment by operator
Ablation temperature (°C)
>85
80–98
Imaging
Real time
Real time
Active cooling system
Yes
Yes
Rectal wall cooling
Yes
Yes
This device uses predefined treatment algorithms with preset energy levels: as a primary procedure, as a re-treatment and for radiation failure. Therefore, the energy level, for each pulse, cannot be individually controlled by the operator. The treatment is planned slice by slice from the apex to bladder neck after the ultrasound scan of the prostate is obtained and reconstructed in 3D. Treatment is delivered to each lobe, anterior to posterior. The transrectal probe is incorporated into a table, which also holds the pump and cooling mechanism. The patient is placed on this table in the right lateral position. In addition, the Ablatherm device has a safety ring that stabilises the rectal wall intraoperatively as well as a patient motion detector [1, 3]. The new Focal One device, which incorporates image fusion, has very similar features to the previous Ablatherm generation and works very much in the same way to deliver the HIFU pulses although it is reported to be more precise.
Primary High-Intensity Focused Ultrasound: Outcomes
Whole-Gland HIFU
Treatment of localised prostate cancer with transrectal HIFU has initially been delivered as a minimally invasive therapy to the entire prostate gland. Historically, HIFU treatment was initially delivered in two sessions, one for each lobe. This was subsequently followed by a single session to treat the whole gland. Some centres combine TURP immediately before the HIFU treatment in a single session, to decrease the gland size, remove intra-prostatic calcification that could impede the ultrasound waves and reduce the risk of obstruction and urinary retention secondary to necrotic tissue (Fig. 10.1a, b).
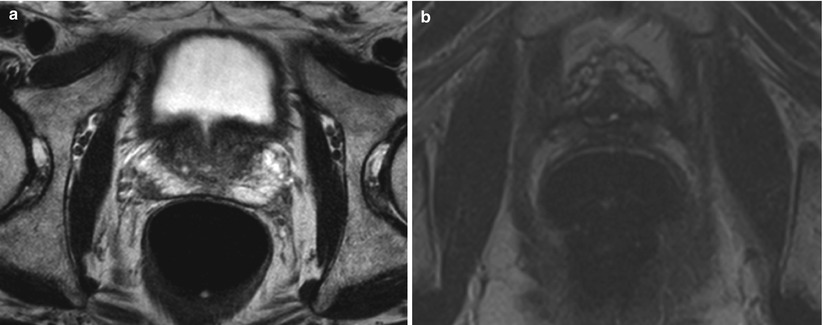

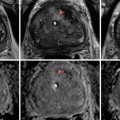
Fig. 10.1
(a) T2-weighted image on the left showing a left peripheral zone lesion. (b) T2-weighted image showing prostatic cavity with whole-gland HIFU ablation
Most HIFU studies have used PSA threshold values and biopsy results to assess failure. Many studies use the ASTRO or Phoenix definitions for biochemical failure. However, biochemical failure is difficult to define with HIFU, more so with focal HIFU.
Poissonnier et al. evaluated 227 patients with localised prostate cancer who were treated with whole-gland HIFU using the Ablatherm device. The HIFU session was combined with a TURP in 176 of these patients. With a follow-up of 27.5 + 20 months, the actuarial disease-free survival rate at 5 years was 66 %. The post-HIFU biopsies carried out after 3 months were negative in 86 % (196 patients). Using PSA nadir as a prognostic value, the patients were divided into three subgroups: nadir PSA <0.5 ng/ml, between 0.51 and 1 ng/ml and >1.1 ng/ml. The negative biopsy rates observed in each subgroup were 89 %, 76 % and 68 %, respectively [5].
The series presented by Blana et al. in 2008 analysed a total of 167 patients who underwent whole-gland HIFU, 116 of which underwent concomitant TURP. Of the 124 patients that had a follow-up biopsy, 115 patients (92.7 %) had no evidence of clinically significant cancer and 12.2 % transitioned into salvage treatment. Grade 1 urinary incontinence was found in ten (6.1 %) of patients, three (1.8 %) patients had grade 2 incontinence and none had grade 3 incontinence. Of 127 patients, 76 had no erectile dysfunction prior to the HIFU procedure. Of these, 42 (55.3 %) had full potency, and 34 (44.7 %) had erectile dysfunction [6].
The multicentre series described by Crouzet et al. followed up a total of 803 patients, of which only 589 patients had biopsies post-HIFU. Of these, control biopsies were negative in 459 patients (77.9 %) and positive in 130 (22.1 %). 22.7 % (182) of patients subsequently had salvage treatment [7]. A further study from the same group followed a total of 1002 patients treated with HIFU to the whole gland with 63 % of patients achieving a PSA nadir of ≤0.3 ng/ml. A total of 37.1 % (371) patients exhibited a PSA rise and transitioned to salvage therapy [8].
Conversely, another French group, Ripert et al., reported on 53 patients with a mean follow-up or 45.4 months and adverse oncological outcomes, although not associated with any risk group. They found that overall, 36 (67.9 %) patients exhibited oncological failure [9].
Similarly, Pfeiffer et al. had a total of 191 consecutive patients and reported overall and cancer-specific survival rates of 86.3 % and 98.4 %, respectively. Metastases were detected in 7 (3.7 %) patients and oncological failure was experienced in 70 (36.6 %) [10].
Thuroff and Chaussy published the outcomes on their 15-year experience. With a total of 704 patients, the cancer-specific and metastases-specific survival rates were 99 % and 95 %, respectively. They report a re-treatment rate of 15 %, urinary incontinence in 4 %, infection in 2.1 % and obstruction in 4.6 % [11].
Similarly, Blana et al. published their long-term results in 2007. In a study incorporating 140 patients, they showed that good cancer control may be achieved with 114 of the 132 (86.4 %) patients who underwent post-HIFU biopsies being negative with no statistical differences between the low- and intermediate-risk groups. The overall survival rates at 5 and 8 years were 90 % and 83 % and only 15 % subsequently had salvage treatment [12]. Further results from the same group a year later with a study of 163 patients showing a disease-free survival rate of 66 % at 5 years with 16 % starting on salvage treatment [6].
These studies showed similar results to their registry analysis of 356 patients from nine centres over a period of 15 years. The rate of negative biopsies in the low-, intermediate- and high-risk groups were 86 %, 78.5 % and 74.2 %, respectively. The median PSA nadir achieved was 0.11 ng/ml at a mean of 14.4 weeks [13].
There are several series that have used the Sonablate device for whole-gland primary HIFU treatment.
Uchida et al. reported on their 5-year experience of whole-gland HIFU, a study incorporating a total of 517 men with T1c-T3N0M0 disease. The biochemical disease-free rate at 5 years was 72 %. Of the 483 who underwent post-HIFU biopsies, 401 (83 %) showed no evidence of clinically significant disease [14]. A further study from the same group focused on the quality of life parameters following HIFU treatment. Of 326 patients, 78 % of patients not receiving neoadjuvant hormonal therapy were potent 24 months after HIFU and the maximum flow rate and residual urine volume returned to baseline values at 12 or 24 months post-HIFU [15].
Another series from Japan looked at 137 patients, achieving a PSA nadir of <1.0 ng/ml was noted in 95 % of patients. Erectile dysfunction occurred in 37 % (22/59) who did not undergo hormonal therapy prior to HIFU. Of 137 patients, 133 underwent post-HIFU prostate biopsies. Of these, six were positive for malignancy [16].
Ahmed et al.’s group reported the first UK series of whole-gland HIFU in 2009. One hundred seventy-two men with a mean follow-up of 346 days showed that 70 % maintained potency by 12 months whilst only 7 % reported mild stress incontinence with no pad use, with a further 0.6 % of patients requiring pads. 92.4 % exhibited no evidence of disease showing that excellent cancer control can be achieved with HIFU [17] (Table 10.2).
Table 10.2
Primary whole-gland HIFU
Study | Device | D’Amico risk groups | Follow-up (months) | Post-HIFU histology | BFSR | Biochemical control | Metastasis-free survival (%) | Cancer-specific survival (%) |
|---|---|---|---|---|---|---|---|---|
Posionner et al. (2006) | Ablatherm N = 227 | Not reported | 27 | 86 % negative | 66 % | 84 % (PSA ≤ 0.5) 7 % (PSA 0.51–1) 8 % (PSA > 1.0) | Not reported | Not reported |
Blana et al. (2007) | Ablatherm N = 140 | Low 51.4 %; inter 48.6 % | 76.8 | 86.4 % negative | 77 % at 5 years 69 % at 7 years | 68.4 % (PSA ≤ 0.5) 31.6 % (PSA > 0.5) | Not reported | Not reported |
Blana et al. (2008) | Ablatherm N = 167 | Low 51.5 %; inter 48.5 % | 57.6 | 92.5 % negative | 75 % | 75 % (Phoenix) 86 % (PSA < 1.0) 64 % (PSA < 0.2) | Not reported | 99.7 % |
Blana et al. (2009) | Ablatherm N = 285 | 56.4 | ||||||
Crouzet et al. (2010) | Ablatherm N = 803 | Low 40.2 %; inter. 46.3 %; high 13.5 % | 42 | 77.9 % negative | 54.3 % (PSA ≤ 0.3) 21.4 % (PSA 0.3–1) 22.3 % (PSA > 1) 1.9 % (not determined) | 97 % | 99 % | |
Ripert et al. (2010) | Ablatherm N = 53 | Low 52.8 %; inter 47.2 % | 45.4 | 21.7 % | 20.8 % (PSA ≤ 0.2) 30.2 % (PSA0.21-1) 49 % (PSA > 1) | Not reported | 100 % | |
Blana et al. (2012) | Ablatherm N = 356 | Low 44.9 %; inter 39.6 %; high 14.6 % | 33.6 | 80.5 % (182/226) negative | 85 % at 5 years 79 % at 7 years | PSA nadir 0.11 at | Not reported | Not reported |
Uchida et al. (2009) | Sonablate N = 517 | Low 28 %; inter 38 %; high 34 % | 24 | 83 % (401/483) negative | 72 % | Not stated | Not reported | Not reported |
Ahmed et al. (2009) | Sonablate N = 172 | Low 28 %; inter 38 %; high 34 % | 12 | 92.4 % negative | Low 76 %; inter 63 %; high 57 % | 61 % (PSA <0.2) 83 % (PSA <0.5) | Not reported | Not reported |
Shoji et al. (2010) | Sonablate N = 326 | Low 84 %; inter 64 %; high 45 % | Not reported | Not reported | ||||
Inoue et al. (2011) | Sonablate N = 137 | Low 21 %; inter 50 %; high 29 % | 36 | 95.5 % negative | Low 91 %; inter 81 %; low 62 % | 95 % (PSA < 1.0) | Not reported | Not reported |
Pfeiffer et al. (2012) | Ablatherm N = 191 | Low 38 %; inter 63.6 %; high 28 % | 52.8 | Low 84.2 %; inter 63.6 %; high 67.5 % total 72.3 % (110/152) negative | Low 84.8 %, inter 64.9 %; low 54.9 % | 74.1 % (PSA < 0.3) | Not reported | 98.4 % |
Thüroff and Chaussy (2013) | Ablatherm N = 704 | Low 21.6 %; inter 38.4 %; high 40 % | 63.6 | 95 % (10 year) | 99 % (10 year) | |||
Crouzet et al. (2013) | Sonablate N = 1002 | Low 37 %; inter 45 %; high 17 %; undefined 1 % | 76.8 | 63 % (485/774) negative | Low 86 %; inter 78 %; high 68 % | 63 % (PSA ≤ 0.3) 56.6 % (PSA ≤ 0.2) | 94 % | 97 % |
Focal HIFU
Focal therapy has emerged as an alternative option to standard treatments. The main aim of focal therapy is tissue preservation with selective ablation, allowing preservation of existing functions and minimising the impact on the quality of life. There are a number of different ablative energies in existence, amongst which is HIFU.
HIFU was first established for use in benign prostatic hyperplasia (BPH). It was then translated for use in localised prostate cancer and one of the first focal HIFU series to be reported was Madersbacher et al.’s study on 29 patients who underwent focal HIFU prior to RP. These men were diagnosed with unilateral localised disease. They showed that focal HIFU was possible without compromising the integrity of intervening structures such as rectum and urethral sphincter [18]. The ability of HIFU to achieve well-defined areas of coagulative necrosis was later confirmed with another study demonstrating the potential for HIFU to treat localised prostate cancer in a focal manner [19] (Figs. 10.2a, b and 10.3a, b).
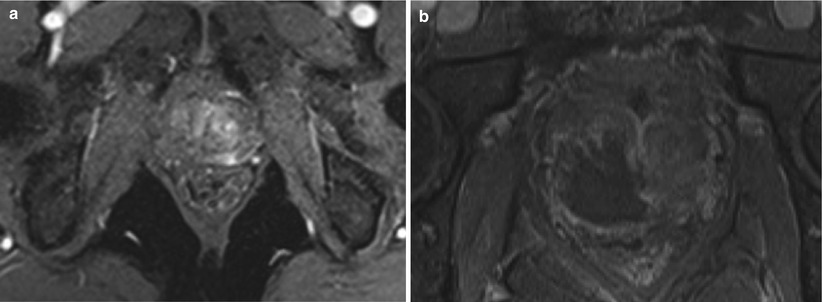
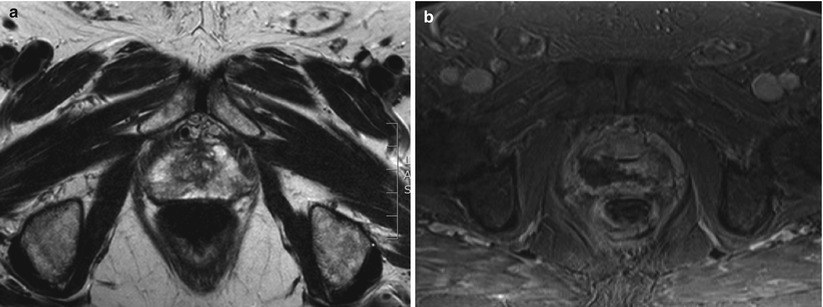

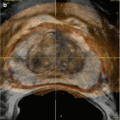
Fig. 10.2
(a) T2-weighted image showing a lesion in the right peripheral zone. (b) T2-weighted image showing treatment of the right peripheral zone lesion with hemiablation HIFU

Fig. 10.3
(a) T2-weighted image showing right peripheral zone lesion. (b) T2-weighted image showing right posterior quadrant ablation HIFU
One of the next series to appear is by Muto et al. where whole-gland and focal HIFU were performed and compared. Indeed, no significant differences were noted in the 2-year biochemical DFS rates for the patients at low and intermediate risk between whole-gland and focal therapy. Interestingly to highlight, there were also no differences noted in the QOL parameters [20]. Indeed, Murat et al. looked at the outcome on erectile dysfunction after 56 patients underwent hemiablation HIFU. For the 52 patients with a pre-HIFU IIEF-5 of more than 17, 28 patients had a post-HIFU IIEF-5 score of more than 17. After the second HIFU session, 20 % of the 15 patients with an IIEF-5 of more than 17 remained with the same outcome [21].
Ahmed et al.’s group have published three series on focal HIFU for localised prostate cancer. The first comes from 2011 as a phase I/II trial, where 20 men were treated with hemiablation HIFU. Ninety-five percent retained their potency whilst 95 % were pad-free. Eighty-nine percent of men achieved the trifecta status of cancer control, erections sufficient for intercourse and pad-free, leak-free continence at 12 months. These results demonstrate the feasibility of hemiablation as one form of focal therapy. However, with the increased precision in the diagnostic pathway of prostate cancer, more precise characterisation has made treating the cancer more focally possible [22].
In fact, the same group published similar findings on 42 men who received focal HIFU with 92 % having no clinically significant cancer on follow-up biopsies in 30/39 men that were biopsied. Four men had re-treatment with no evidence of disease on MRI at 12 months. They report 89 % of men having sufficient erections for penetration, and all men with no baseline incontinence achieved pad-free continence at 12 months [23].
The third also reported similar findings. A total of 56 patients were treated with focal HIFU, of which 85.7 % had no measurable prostate cancer post-HIFU. Forty patients were leak-free, pad-free and had erections sufficient for penetration. Of 41 patients with good baseline function, 22 (53.7 %) achieved the trifecta status of pad-free, leak-free continence, good erectile function and absence of clinically significant disease [24].
El Fegoun et al. published the first series on focal HIFU in the elderly patient cohort. Hemiablation HIFU was performed in a total of 12 patients, 11 of whom had negative follow-up biopsies. One patient had re-treatment with HIFU and 4 were subsequently treated with androgen-deprivation therapy. The recurrence-free survival rate was 90 % at 5 years. Low morbidity rates are reported: none experience urinary incontinence, one patient suffered from an episode of acute urinary retention, and two had a urinary tract infection [25].
Targeting treatment to a discrete area of prostate tissue preserves surrounding structure and in doing so helps maintain patients’ functions and quality of life. Focal HIFU has recently emerged as one of the minimally invasive techniques that provide acceptable morbidity rates whilst giving good oncological outcomes (Table 10.3).
Table 10.3
Primary focal HIFU
Study | Device | D’Amico risk groups | Mean follow-up | Post-HIFU histology (%) | BDFS | PSA kinetics | Secondary treatment actual (%) | Metastases-specific survival (%) | Cancer-specific survival (%) |
|---|---|---|---|---|---|---|---|---|---|
Madersbacher et al. (1995) | Sonablate N = 29 | NR | NR | NR | NR | NR | NR | NR | NR |
Beerlage et al. (1999) | Ablatherm N = 14 | NR | NR | 28 % negative | NR | 19 % (PSA < 0.5) 50 % (PSA0.5-4.0) 30 % (PSA > 4) | NR | NR | NR |
Muto et al. (2008) | Sonablate N = 29 | Low 86.2 %; inter13.8 % | 32 median | 76.5 % negative | Low 83.3 % inter 53.6 % | NR | NR | NR | |
Murat et al. (2009) | NR N = 56 | NR | 42 median | NR | 76 % (3 years) 60 % (5 years) | PSA nadir 0.8 After 1st HIFU PSA nadir 0.47 After 2nd HIFU | NR | NR | NR |