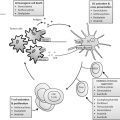
Fig. 4.1
A schematic diagram of the physical principle of HIFU for noninvasive ablation. a HIFU transducer, focusing ultrasound energy into a small volume while ultrasound beams transmit through the overlying tissue of a targeted breast cancer. b Normal breast tissue. c A targeted breast cancer
Two major effects are directly involved in the tissue damage induced by HIFU exposure. The first is a thermal effect from the conversion of mechanical energy into heat in the tissue, and the second is through cavitation. The thermal effect depends on the temperature achieved and the length of HIFU exposure. If the temperature rise is above a threshold of 56 °C and the exposure time is 1 s [25],irreversible cell death will be induced through coagulation necrosis. In fact, the temperature at a focal volume may rise rapidly above 80 °C during HIFU treatments [26].A steep temperature gradient is detected between the focus and normal non-focal surrounding tissue, and therefore sharp demarcation between the treated and untreated tissue is demonstrated in histological examination.
The second mechanism is acoustic cavitation [27].Acoustic cavitation can be defined as the interaction of a sound field with the microscopic gas bodies in a sonicated medium. The presence of small gaseous nuclei existing in subcellular organelles and fluid in tissue are the source of cavitation, which can expand and contract under influence of the acoustic pressure. During the collapse of bubbles, the acoustic pressure is more than several thousand pascals, and the temperatures reach several thousand degrees Celsius [28]. Therefore, it may cause tissue damage that is less predictable than the effect on tissue shape and position caused by heating [29].However, recent experimental studies have been investigating the idea of promoting cavitation for enhancing the level of ablation and reducing required exposure time [30].This is in contrast to previous approaches, where cavitation was viewed as an unpredictable damage mechanism that should be avoided [31].
Thermal ablation can cause direct and indirect damages to a targeted tumor. Direct and indirect heat injury occurs during the period of heat deposition, and it is predominately determined by the total energy delivered to a targeted tumor [32]. Indirect injury usually occurs after thermal ablation, which produces a progression in tissue damage . It may involve a balance of several factors including microvascular damage, cellular apoptosis, Kupffer cell activation, altered cytokine release and antitumor immune response [33]. Direct injury is generally better defined than the secondary indirect effects.
4.3 Technical Aspects of HIFU Ablation
The volume of tissue ablation induced with one HIFU exposure can be regarded as a lesion. The focal volume of HIFU transducer is usually ellipsoid or cigar-shaped, with dimensions of 10–20 mm along the beam axis and 1–2 mm in the transverse direction. Therefore, the lesion induced with single HIFU exposure is usually very small. But, while lesions are placed side by side, confluent volumes of ablation can be achieved.
To ablate clinically relevant volumes of tissue for the treatment of carcinomas, many of these small lesions should be positioned side by side systematically to “paint out” or cover a targeted volume, without any remaining normal tissue between each lesion. If a suitable therapy plan is correctly performed, HIFU is able to ablate various shapes and sizes of solid tumors in a conformal fashion.
As a result, HIFU therapy can be clinically defined as a precise procedure to ablate an entire tumor by moving high-energy concentrated focus side by side in a 3-D fashion. At the beginning of HIFU procedure, the targeted tumor is identified and divided into parallel slices. Using HIFU exposure regimes, the tumor on each slice is completely ablated, and this process is repeated slice by slice to achieve complete coagulation of the targeted tumor,as shown in Fig. 4.2.
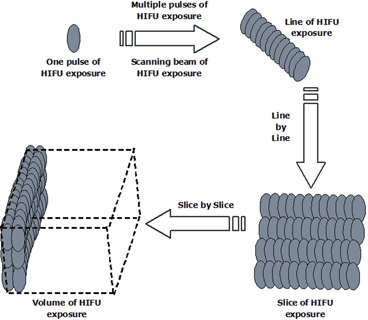

Fig. 4.2
Schematic diagram showing the HIFU conformal therapeutic plan, which is used to ablate the whole volume of a targeted tumor
A single exposure can be made to induce a cigar-shaped lesion when the location of the focal volume is immoveable. The exposure time for each pulse ranges from one second to several seconds. The single exposure can be repeated at predetermined intervals in the same position. Multiple single exposures can produce a line-shaped lesion through placing single-exposure lesions side by side with a present overlap and with a predetermined time interval between exposures. The multiple single exposures can be repeated in the perpendicular direction to form a slice-shaped lesion. A line-shaped lesion can also be achieved by a linear track exposure in which the activated transducer is moved at a constant speed over a line. This may be made by traversing one or more times in one direction only, or by scanning in both directions (“there and back”) without pausing at the furthest extremity. Several of these tracks may be superimposed in one exposure period at chosen (preset) time intervals. Superimposition of tracks leads to an increase in the extent of ablation in the direction perpendicular to the direction of motion due to thermal conduction. As a result, a slice-shaped lesion can be induced in this way. From one slice-shaped lesion to next ones, confluent volumes of ablation can be achieved.
The selection of the above exposure regimes during HIFU procedure is very complicated in clinical practice. It depends on the component of overlying tissue structure, acoustic window, the depth of tumor from the skin, vital structures surrounding tumor, tumor vascularity and size. For instance, single exposure and a linear scan exposure can be chosen for the treatment of a superficial poorly vascularised tumor. However, multiple single exposures are usually used in the treatment of deep vasculiarised tumors. In clinics they can be separately used for an individual patient simultaneously. Therefore, only doctors with a knowledge base from a specialized training course could perform this treatment. The experience that doctors have and reasonable judgment that they can make during HIFU procedure are important at the early stage of HIFU clinical application. As therapeutic data are extensively collected for each type of solid malignancy, improvement is expected in the near future.
4.4 Direct Thermal and Non-thermal Effects on the Tumor
The effects of thermal ablation on a targeted tumor are determined by temperature increase, thermal energy deposited, rate of heat removal, and the specific thermal sensitivity of the tissue. As the tissue temperature rises, the time required to achieve irreversible cellular damage decreases exponentially. At temperatures between 50–55 °C, cellular death occurs instantaneously in cell culture [34]. Protein denaturation, membrane rupture, cell shrinkage, pyknosis and hyperchromasia occur ex vivo between 60 and 100 °C, leading to immediate coagulation necrosis [35]. Tissue vaporization and boiling are superimposed on this process at temperatures higher than 105 °C. Carbonization, charring and smoke generation occur while the temperature is over 300 °C [36].
In addition, acoustic cavitation , one of the mechanical effects induced by HIFU ablation, is the most important non-thermal mechanism for tissue disruption in ultrasound field [27]. The presence of small gaseous nuclei existing in subcellular organelles and fluid in tissue are the source of cavitation, which can expand and contract under influence of the acoustic pressure. During the collapse of bubbles, the acoustic pressure and subsequently high temperature can induce the local destruction of a targeted tissue [28, 29].
4.5 Thermal Effects on Tumor Vasculature
Structural and functional changes are directly observed in tumor vasculature after thermal ablation. These changes are not as well described as thermal effects on the tissues, but they rely on varying temperatures. At temperatures between 40 and 42 °C, there is no significant change in tumor blood flow after 30–60 min exposure [37]. Beyond 42–44 °C, there is an irreversible decrease in tumor blood flow, with vascular stasis and thrombosis, resulting in heat trapping and progressive tissue damage [38]. While temperatures exceed 60 °C, immediate destruction of tumor microvasculature occurs [39]. It cuts the blood supply to the tumor directly through the cauterization of the tumor feeder vessels, leading to deprivation of nutrition and oxygen. Thus, tissue destruction can be enhanced by the damage caused by thermal ablation to tumor blood vessels.
4.6 Indirect Effects on the Tumor
Indirect injury is a secondary damage to tissue, which progresses after the cessation of thermal ablation stimulus [33]. It is based on histological evaluation of tissue damage at various time points after thermal ablation. The full extent of the secondary tissue damage becomes evident 1–7 days after thermal ablation , depending on the model and energy source used [40, 41]. The exact mechanism of this process is still unknown. However, it may represent a balance of several promoting and inhibiting mechanisms, including induction of apoptosis , Kupffer cell activation, and cytokine release.
Cellular apoptosis may contribute to the progressive injury of tissue after thermal ablation. It is well-established that apoptosis increases in a temperature-dependent manner, and temperatures between 40 and 45 °C cause inactivation of vital enzymes, thus initiating apoptosis of tumor cells [42, 43]. Most thermal ablation techniques create a temperature gradient that progressively decreases away from the site of probe insertion. The induction of apoptosis at a distance from the heat source may potentially contribute to the progression of injury. Increased rate of apoptosis is observed in the liver 24 h after microwave ablation. The stimulation of apoptosis may be directly induced by temperature elevations, alterations in tissue microenvironment, and the release of various cytokines after thermal ablation.
Kupffer cell activity may be one of the major factors involved in the progressive injury after thermal ablation [34]. Heat triggers Kupffer cells to secret IL-1 [44] and tumor necrosis factor-α (TNF-α) [45], which are known to have in vivo antitumor activity [46] and to increase apoptosis in cancer cells [43]. Kupffer cells also induce the production of interferon that augments the liver-associated natural killer cell activity [47].
Thermal ablation may induce both regional and systemic production of cytokines through activation of inflammatory cells. Compared with controls, the circulating level of IFN-γ and vascular endothelial growth factor levels markedly increase after RFA [48, 49]. The increased level of IL-1 and TNF-α is also observed after RFA [50]. These cytokines may have direct cytotoxic effects such as inducing tumor endothelial injury and rendering tumor cells more sensitive to heat-induced damage [51, 52]. However, contrasting results are obtained for TNF-α level in two studies [49, 53] and IL-1 level in one study [54], which remains unchanged after thermal ablation.
4.7 HIFU -Induced Antitumor Immune Response
It has been noted that large amounts of tumor debris remain in situ after thermal ablation. As a normal process of healing response, the tumor debris is gradually reabsorbed by the individual patient, which takes a period ranging from months to a few years. It is still unclear what kind of biological significance may exist during the absorption of the ablated tumor. However, some studies have shown that active immune response to the treated tumor could be developed after thermal ablation, and the host immune system could become more sensitive to the tumor cells [55–58]. This may lead to a potential procedure that reduces or perhaps eliminates metastases , and prevents local recurrence in cancer patients who have had original dysfunction of antitumor immunity before treatment.
Animal studies have suggested that HIFU may modulate host anti-tumor immunity. Yang and colleagues [59] used HIFU to treat C1300 neuroblastoma implanted in mouse flanks, followed by the re-challenge of the same tumor cells. A significantly slower growth of re-implanted tumors was observed in these mice while compared with the controls. After HIFU treatment, the cytotoxicity of peripheral blood T-lymphocytes was significantly increased in the H22 tumor bearing mice treated with HIFU, and adoptive transfer of the activated-lymphocytes could provide better long-term survival and lower metastatic rates in the mice re-challenged by the same tumor cells [55]. Similar results were confirmed in the mice implanted MC-38 colon adenocarcinoma after HIFU ablation. HIFU treatment could also induce an enhanced CTLs activity in vivo, thus provides protection against subsequent tumor re-challenge [60].
After HIFU ablation, large amounts of tumor debris remain in situ,and the host gradually reabsorbs them as the normal process of a healing response. Using a murine hepatocellular carcinoma model, Zhang and colleagues [61] demonstrated that the remaining tumor debris induced by HIFU could be immunogenic as an effective vaccine to elicit tumor-specific immune responses, including induction of CTL cytotoxic activity and protection against a lethal tumor challenge in naïve mice. When the tumor debris was loaded with immature DCs, it could significantly induce maturation of DCs, and increased cytotoxicity and TNF-α and IFN-γ secretion by CTL, thus initiating host specific immune response after H22 challenge in the vaccinated mice [62]. Immediately after HIFU exposure to MC-38 colon adenocarcinoma cells in vitro, the release of endogenous danger signals including HSP60 was observed from the damaged cells. These signals could subsequently activate APCs, leading to an increased expression of co-stimulatory molecules and enhanced secretion of IL-12 by the DCs, and elevated secretion of TNF-a by the macrophages [63]. In addition, HIFU could upregulate in vitro and ex vitro molecule expression of HSP70 [64, 65], which are intracellular molecular chaperones that can enhance tumor cell immunogenicity, resulting in potent cellular immune responses.
The potency of APCs activation from mechanical lysis and a sparse-scan HIFU was much stronger than that from thermal necrosis and a dense-scan HIFU exposure, suggesting that optimization of HIFU ablation strategy may help to enhance immune response after treatment [66]. Heat and acoustic cavitation are two major mechanisms involved in HIFU-induced tissue damage , and cavitation is a unique effect of HIFU while compared with other thermal ablation techniques. It causes membranous organelles to collapse, including mitochondria and endoplasmic reticulum, cell and nuclear membrane. This breaks up tumor cells into small pieces, on which the tumor antigens may remain intact, or leading to the exposure of an immunogenic moiety that is normally hidden in tumor antigens [55]. Zhou and colleagues [67] used either heat- or HIFU-treated H22 tumor vaccine to inoculate naïve mice. The vaccination times were four sessions, once a week for 4 consecutive weeks, and each mouse was challenged with H22 tumor cells 1 week after the last vaccination. They found that the HIFU-treated tumor vaccine could significantly inhibit tumor growth, and increase survival rates in the vaccinated mice, suggesting that acoustic cavitation could play an important role to stimulate host antitumor immune system.
Emerging clinical results revealed that systemic cellular immune response was observed in cancer patients after HIFU treatment. Rosberger and colleagues [68] reported five consecutive cases of posterior choroidal melanoma treated with HIFU . Three patients had abnormal, and two patients normal CD4/CD8 ratios before treatment. One week after treatment, the ratio in two patients reverted to normal, while another was noted to have a 37 % increase in his CD4 T-cells relative to his CD8 cells. Wang and Sun [69] used multiple-session HIFU to treat 15 patients with late-stage pancreatic cancer. Although there was an increase in the average values of NK cell and T lymphocyte and subset in ten patients after HIFU treatment, a significant statistical difference was observed in only NK cell activity before and after HIFU treatment (p < 0.05). Wu and colleagues [70] observed changes in circulating NK, T lymphocyte and subsets in 16 patients with solid malignancy before and after HIFU treatment. The results showed a significant increase in the population of CD4+ lymphocytes (p < 0.01) and the ratio of CD4+/CD8+ (p < 0.05) after HIFU treatment. The abnormal levels of CD3+ lymphocytes returned to normal in two patients, CD4+/CD8+ ratio in three, CD19+ lymphocytes in one, and NK cell in one, respectively, in comparison to the values in the control group. In addition, serum levels of immunosuppressive cytokines including VEGF, TGF-β1 and TGF-β2 were significantly decreased in peripheral blood of cancer patients after HIFU treatment, indicating that HIFU may lessen tumor-induced immunosuppression , and renew host antitumor immunity [71].
Clinical evidences suggest that HIFU treatment may also enhance local antitumor immunity in cancer patients. Kramer and colleagues [72] found that HIFU treatment could alter the presentation of tumor antigens in prostate cancer patients, which was most likely to be stimulatory. Histological examination showed significantly upregulated expression of HSP72, HSP73, and glucose regulated protein (GRP) 75 and 78 at the border zone of HIFU treatment in prostate cancer. Heated prostate cancer cells exhibited increased Th1-cytokine (IL-2, IFN-γ, TNF-α) release but decreased Th2-cytokine (IL-4, -5, -10) release of TILs. The upregulated expression of HSP70 was confirmed in the tumor debris of breast cancer after HIFU ablation [73], indicating that HIFU may modify tumor antigenicity to produce a host immune response . Xu and colleagues [74] found the number of tumor-infiltrating APCs including DCs and macrophages increased significantly along the margin of HIFU-treated human breast cancer, with an increased expression of HLA-DR, CD80 and CD86 molecules. Activated APCs may take up the HSP-tumor peptide complex, which remains in the tumor debris, and present the chaperoned peptides directly to tumor-specific T lymphocytes with high efficiency, resulting in potent cellular immune responses against tumor cells after HIFU treatment. Furthermore, HIFU could induce significant infiltration of TILs in human breast cancer, including CD3, CD4, CD8, B lymphocytes and NK cells. The numbers of the activated CTLs expressing FasL+, granzyme+ and perforin+ significantly increased in the HIFU-treated tumor, suggesting that specific cellular antitumor immunity could be locally triggered after HIFU treatment [75].
4.8 Conclusions
HIFU therapy for solid malignancies has been mostly conducted in research settings for the assessment of technical safety, efficacy and feasibility. It provides a non-invasive therapeutic option that once perfected will add a useful extra string to the clinicians bow. There is clearly a place for HIFU ablation because common and serious problems affect many thousands of people every year in the clinical management of solid malignancies. If HIFU can offer an option to even a small proportion of these patients, it is vital to continue pushing the technology forward. Where clinically appropriate, HIFU should give at least the same results as surgical excision, with the extent of the negative surgical margins. Although recent results have been very encouraging, multiple-central, long-term follow-up trials are essential to evaluate the long-term efficacy and cost-effectiveness of HIFU treatments in cancer. Not until these issues have been resolved, and the results from prospective, randomized clinical trials worldwide become available, can this noninvasive ablative technique be considered as a candidate for conventional therapy for widespread clinical applications.
Beyond optimization of technical and physiological parameters, it is clear that HIFU ablation should be undertaken when there is precise knowledge not only of the number and location of the lesions, but also of the biological characteristics and natural history of the tumor. The goal of tumor therapy is that all cancer cells should be completely killed in the patient’s body. A similar multidisciplinary approach including other modalities is important in the treatment of solid malignancies. For patients with cancer , the therapeutic strategy for the disease should be a multiple treatment plan, which includes local treatments such as surgery and radiotherapy, and systemic therapy such as chemotherapy and immunotherapy . So, success achieved in the application of HIFU treatment is mainly dependent not only on the HIFU technique, but also on better understanding of the natural characteristics of tumors.
Recent studies supports academic evidence that thermal ablation may elicit a systemic antitumor immune response. They range from anecdotal observations in a clinical setting, a variety of animal models and correlative immune studies in patients undergoing thermal ablation. It is not surprising that there is great concern about a close relationship between thermal ablation and antitumor immune response as thermal ablation may has the potential to be both local and systemic therapies. It may lead to a post-ablative procedure that reduces or perhaps eliminates distant disease, and prevents local recurrence through the immune system in cancer patients who have had original dysfunction of antitumor immunity after ablation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree