The authors review the course and appearance of the major segments of the upper cranial nerves from their apparent origin at the brainstem through the proximal extraforaminal region, focusing on the imaging and anatomic features of particular relevance to high-resolution magnetic resonance imaging evaluation. Selected pathologic entities are included in the discussion of the corresponding cranial nerve segments for illustrative purposes.
Key points
- •
High-resolution isotropic three-dimensional (3D) magnetic resonance imaging acquisition relying heavily on constructive interference in the steady state (CISS) with and without contrast has largely replaced 2D techniques previously used for the evaluation of the cranial nerves (CNs) at the authors’ institution and allows for high-contrast evaluation of the CNs along a greater portion of their extent than was previously possible.
- •
The cisternal and dural cave segments of the upper CNs, with the possible exception of the fourth cranial nerve, are well evaluated with noncontrast CISS.
- •
The interdural and foraminal segments of the CNs are revealed after the administration of intravenous contrast.
- •
The proximal extraforaminal segments of the CNs are often well visualized on 3D high-resolution imaging with CISS.
- •
Evaluation for pathologic contrast enhancement in segments of the upper CNs which were previously not well seen, and on a scale previously not well depicted, is now possible with high-resolution 3D technique.
Introduction
Although long used for the evaluation of the course of the cranial nerves (CNs) within the subarachnoid space, the recognition of the utility of enhancement on postcontrast constructive interference in the steady state (CISS) imaging allows for the evaluation of the remainder of the extended course of the CNs once they exit the subarachnoid space. In this article, the authors review the recent literature on the evaluation of the CNs with CISS and analogous sequences and describe the use of such techniques for the evaluation through the extraforaminal segments. In many instances, such techniques enable visualization of nearly the entire course of many of the upper CNs, and the relevant anatomy is described here following the segmental classification suggested in the article by Blitz, Choudhri, Chonka and colleagues within this issue ( Box 1 ).
- a.
Nuclear
- b.
Parenchymal fascicular
- c.
Cisternal
- d.
Dural cave
- e.
Interdural
- f.
Foraminal
- g.
Extraforaminal
CISS imaging combines true and reversed fast imaging in steady state precession (FISP) imaging to allow for high-signal, high-spatial resolution imaging with suppression of banding artifacts caused by unbalanced imaging gradients and field inhomogeneities. CISS is a gradient echo technique, and images acquired have the appearance of strong T2 weighting but, in fact, demonstrate some degree of mixed weighting, which allows for the visualization of enhancement.
The application of precontrast and postcontrast CISS imaging has several significant advantages over other techniques used for cranial nerve evaluation. CISS imaging allows for the acquisition of high spatial resolution isotropic 3-dimensional (3D) imaging in a clinically acceptable time period, allowing for the evaluation of the entirety of the skull base and posterior fossa at 0.6-mm isotropic resolution in less than 6 minutes. The addition of contrast allows for the depiction of the CNs in most segments with a single sequence, and it is simultaneously possible to assess for pathologic contrast enhancement. Unless otherwise noted, the images in this article were acquired on a 3T Siemens Verio (Ehrlangen, Germany) with 0.6-mm isotropic resolution.
Although for the purposes of this discussion the authors use the nomenclature established in the article by Blitz, Choudhri, Chonka and colleagues within this issue for CN I to VI, CN I and CN II are properly considered tracts of the central nervous system (CNS) rather than true cranial nerves passing into the peripheral nervous system (PNS) and do not entirely conform to the same anatomic outline as CN III to VI. Although classically anatomists describe nerves from their point of origination to their destination, the mixed afferent and efferent components of several cranial nerves makes this challenging and potentially confusing. For the sake of simplicity, descriptions are given here following the nerve from their point of apparent origin at the brainstem peripherally into the extraforaminal components.
CN dysfunction may arise from pathologic conditions interrupting fiber bundles at any point from the CN nuclei to the end organ innervated by the nerve or often even from lesions within pathways that themselves innervate the CN nuclei. Lesions within the brain, cranial nerve nuclei, or fiber tracts, as they course to exit the brain and give rise to the CNs, are often associated with other signs of damage to the CNS ; such lesions are discoverable on imaging of the brain. Imaging of the cranial nerve nuclei and course of fibers within the substance of the brainstem itself is beyond the scope of this discussion, which focuses on imaging the CNs from their apparent origin at the surface of the brainstem through their exit from the skull base foramina. The interested reader will find excellent information on the location and course of the nuclear and fascicular segments, for instance, in the recent text by Naidich and colleagues. Additionally, it is important to note that the entirety of the extraforaminal course of the upper CNs is complex and limited space precludes treatment of the entirety of the topic beyond some introductory remarks within this article.
Introduction
Although long used for the evaluation of the course of the cranial nerves (CNs) within the subarachnoid space, the recognition of the utility of enhancement on postcontrast constructive interference in the steady state (CISS) imaging allows for the evaluation of the remainder of the extended course of the CNs once they exit the subarachnoid space. In this article, the authors review the recent literature on the evaluation of the CNs with CISS and analogous sequences and describe the use of such techniques for the evaluation through the extraforaminal segments. In many instances, such techniques enable visualization of nearly the entire course of many of the upper CNs, and the relevant anatomy is described here following the segmental classification suggested in the article by Blitz, Choudhri, Chonka and colleagues within this issue ( Box 1 ).
- a.
Nuclear
- b.
Parenchymal fascicular
- c.
Cisternal
- d.
Dural cave
- e.
Interdural
- f.
Foraminal
- g.
Extraforaminal
CISS imaging combines true and reversed fast imaging in steady state precession (FISP) imaging to allow for high-signal, high-spatial resolution imaging with suppression of banding artifacts caused by unbalanced imaging gradients and field inhomogeneities. CISS is a gradient echo technique, and images acquired have the appearance of strong T2 weighting but, in fact, demonstrate some degree of mixed weighting, which allows for the visualization of enhancement.
The application of precontrast and postcontrast CISS imaging has several significant advantages over other techniques used for cranial nerve evaluation. CISS imaging allows for the acquisition of high spatial resolution isotropic 3-dimensional (3D) imaging in a clinically acceptable time period, allowing for the evaluation of the entirety of the skull base and posterior fossa at 0.6-mm isotropic resolution in less than 6 minutes. The addition of contrast allows for the depiction of the CNs in most segments with a single sequence, and it is simultaneously possible to assess for pathologic contrast enhancement. Unless otherwise noted, the images in this article were acquired on a 3T Siemens Verio (Ehrlangen, Germany) with 0.6-mm isotropic resolution.
Although for the purposes of this discussion the authors use the nomenclature established in the article by Blitz, Choudhri, Chonka and colleagues within this issue for CN I to VI, CN I and CN II are properly considered tracts of the central nervous system (CNS) rather than true cranial nerves passing into the peripheral nervous system (PNS) and do not entirely conform to the same anatomic outline as CN III to VI. Although classically anatomists describe nerves from their point of origination to their destination, the mixed afferent and efferent components of several cranial nerves makes this challenging and potentially confusing. For the sake of simplicity, descriptions are given here following the nerve from their point of apparent origin at the brainstem peripherally into the extraforaminal components.
CN dysfunction may arise from pathologic conditions interrupting fiber bundles at any point from the CN nuclei to the end organ innervated by the nerve or often even from lesions within pathways that themselves innervate the CN nuclei. Lesions within the brain, cranial nerve nuclei, or fiber tracts, as they course to exit the brain and give rise to the CNs, are often associated with other signs of damage to the CNS ; such lesions are discoverable on imaging of the brain. Imaging of the cranial nerve nuclei and course of fibers within the substance of the brainstem itself is beyond the scope of this discussion, which focuses on imaging the CNs from their apparent origin at the surface of the brainstem through their exit from the skull base foramina. The interested reader will find excellent information on the location and course of the nuclear and fascicular segments, for instance, in the recent text by Naidich and colleagues. Additionally, it is important to note that the entirety of the extraforaminal course of the upper CNs is complex and limited space precludes treatment of the entirety of the topic beyond some introductory remarks within this article.
CN I: olfactory nerve
The olfactory nerve provides the sense of smell. The portions of the olfactory nerve visible on magnetic resonance (MR) imaging, namely, the olfactory bulb (OB) and olfactory tract (OT), are not components of a cranial nerve in the formal sense but rather a forward extension of the telencephalon, more properly a tract of the CNS. As such, CN I does not follow the typical segmental pattern of CNs III to XII. For the purposes of this discussion, the authors define cisternal, foraminal, and extraforaminal segments. The olfactory tracts carry information on smell from the cisternal segment into the olfactory stria to the primary olfactory cortex located in the inferomedial aspect of the temporal lobe.
CN I.c (Cisternal): Anatomy
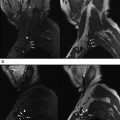
CN I.c consists of the OB and the OT ( Fig. 1 ). The OB sits above the cribriform plate and receives input from primary sensory neurons. The CNS-PNS transitional zone (TZ), located in the OB, is histologically distinct from that of the other CNs with a cell type, the ensheathing cell, not found in other CNs. The OB is uniformly well visualized with CISS. The neurons of the OB project posteriorly into the OT, which in turn extends posteriorly toward the brain. The OT is distinguished from the OB by a change in caliber, although the lack of a constant landmark has led to variability in identification between subjects and readers, hampering clinical evaluation and research. Some investigators advocate spin echo imaging rather than CISS for the evaluation of CN I.c to avoid artifacts in this region caused by air tissue inferface. In the authors’ experience, diagnostic images of the anterior cranial fossa and paranasal sinuses can be obtained with CISS, with the significant advantage of also allowing for the assessment of contrast enhancement at a high spatial resolution. The quantitative size evaluation of OB and OT has demonstrated a correlation between OB/OT volume and olfactory function and an age-related decrease in OT volume.
CN I.c: Selected Pathologic Conditions
The congenital absence of CN I.c may be detected in patients as a component of Kallmann syndrome. Idiopathic olfactory loss is common, and patients with idiopathic olfactory loss demonstrate decreased OB volumes compared with normal controls. Idiopathic anosmia can be a harbinger of Parkinson disease or Alzheimer disease. Intra-axial and extra-axial tumors, most frequently meningioma, arising from the anterior skull base can result in anosmia. The vulnerability of the CN I.c to head trauma and posttraumatic olfactory dysfunction is well documented and also correlates with OB/OT size.
CN I.f (Foraminal): Anatomy
Up to 100 unmyelinated axons of the olfactory neuroepithelium form bundles (olfactory fila, the olfactory nerves proper) (see Fig. 1 B white arrows) that extend intracranially through the openings of the cribriform plate before penetrating the dura. The numerous fila and the foramina in the cribriform plate are not reliably visualized with the current imaging resolution.
CN I.f: Selected Pathologic Conditions
Traumatic injury to the cribriform plate resulting in loss of olfaction or cerebrospinal fluid (CSF) leak (CSF rhinorrhea) may occur in this region.
CN I.g (Extraforaminal): Anatomy
The primary olfactory neurons are located in the neuroepithelium lining the superior nasal cavity.
CN I.g: Selected Pathologic Conditions
The most common cause of anosmia involving the CN I.f is rhinosinusitis. The prototypical tumor of the CN I.g is esthesioneuroblastoma (olfactory neuroblastoma), a rare lesion arising from the olfactory epithelium. A variety of tumors typically arise at the anterior skull base and in the region of CN I.g that commonly extend superiorly toward CN I.f. One of the main surgical considerations for malignant sinonasal tumors, including esthesioneuroblastomas, is whether the tumor has extended intracranially, which has an impact on the surgical approach as well as the prognosis. CISS imaging provides a very accurate identification of the intracranial extension of disease and may allow the radiologist to detect the possibility of even a very small amount of intracranial tumor ( Fig. 2 ).
CN II: optic nerve
The optic nerve carries visual information from the globes into the intracranial compartment with a partial decussation of fibers at the optic chiasm and synapses at the lateral geniculate nucleus of the thalamus. As noted earlier, CN II is not a cranial nerve by the strict definition and does not conform to the segmental classification described. For the purposes of this discussion, the authors divide the nerve into cisternal, dural cave, foraminal, and extraforaminal segments. All of these CN II segments are intradural; however, the surrounding tissue signal varies from CSF to bone and fat. For the purposes of this discussion, the authors define the attached component of CN II, the optic chiasm and optic tracts, as the fascicular component extending toward the lateral geniculate nucleus posterolaterally. As with the other CNs, the cisternal segment is limited to the component surrounded by the subarachnoid space on all sides. High-resolution CISS imaging provides superb anatomic detail regarding the location of CN II and its relationship to surrounding structures; postcontrast CISS imaging may also demonstrate subtle contrast enhancement within the nerve. For the assessment of intrinsic T2 signal abnormality, such as seen in optic neuritis, reliance on CISS technique alone is not advised, and imaging can be supplemented with spin echo–based T2 techniques.
CN II.c (Cisternal): Anatomy
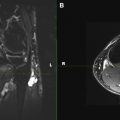
For the purposes of this article, the cisternal anatomy of the optic nerves is limited to the portion of the prechiasmatic optic nerve, which extends from the posterior margin of the anterior clinoid process posteromedially to the optic chiasm ( Fig. 3 ).
CN II.c: Selected Pathologic Conditions
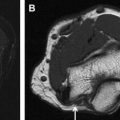
The most commonly encountered pathologic condition of CN II.c in adults is caused by extrinsic compression by masses, such as pituitary macroadenoma, meningioma, or craniopharyngioma. The location of CN II.c in relation to the mass ( Fig. 4 ) is of critical importance for procedural planning and is often clarified with the use of high-resolution imaging, such as CISS or fast imaging employing steady-state acquisition. Intrinsic masses of the nerve, such as optic nerve gliomas, are most commonly encountered in the pediatric population. Because the entirety of CN II is within the CNS, no Schwann cells exist to give rise to schwannomas.
CN II.d (Dural Cave): Anatomy
The definition of the dural cave segment of CN II is somewhat arbitrary, but it does exist and may be seen as a CSF-filled recess partially surrounding the optic nerve before it emerges from the optic canal. The anterior border of CN II.d is difficult to define anatomically but may be construed on MR imaging as the point beyond which no CSF signal is visible. The posterior border of CN II.d is at the posterior margin of the anterior clinoid process.
CN II.f (Foraminal): Anatomy
The optic nerves pass through the skull base via the optic canals (see Fig. 3 ), which are bounded by the anterior clinoid processes laterally and the orbital plates of the ethmoid bones medially. CN II.f may at times be difficult to evaluate on CISS because of susceptibility artifacts created by bone and air in the adjacent paranasal sinuses and sometimes pneumatized anterior clinoid process.
CN II.f: Selected Pathologic Conditions
Masses in this region are often small and comparatively difficult to visualize. Meningioma is the most common mass lesion affecting CN II.f. Primary and secondary bony processes involving the anterior clinoid process and inflammatory and neoplastic processes involving the paranasal sinuses may also secondarily affect CN II.f.
CN II.g (Extraforaminal): Anatomy
The optic nerve is visible within the orbit (see Fig. 3 B) and is separated from the orbital fat by the optic nerve sheath, which contains fluid similar to CSF and variably communicates with the subarachnoid space.
CN II.g: Selected Pathologic Conditions
A myriad of neoplastic and non-neoplastic mass lesions may affect CN II.g. In case of intraorbital masses associated with the CN II.g, it is crucial for differential diagnosis and surgical planning to know whether the mass is originating from the optic nerve itself, the optic nerve sheath, or surrounding structures; whether the optic nerve is encased or displaced by tumor; and what the relative position of the nerve is compared with the mass. Postcontrast CISS imaging is often helpful in this regard because it shows the nerve against the enhancing tumor. Because the fat signal is not suppressed in CISS, the addition of high-resolution, postcontrast, fat-suppressed, T1-weighted images can be helpful in demonstrating enhancing tumor/orbital fat interface.
CN III: oculomotor nerve
The oculomotor nerve has both a motor function, innervating most of the muscles associated with the orbit, as well as a parasympathetic component that functions to constrict the pupil. The nuclei of the oculomotor nerve are located in the dorsal aspect of the midbrain just ventral to the cerebral aqueduct at the midline. From the nuclei, fibers course anteriorly and slightly laterally to exit the ventral aspect of the brainstem.
CN III.c (Cisternal Course): Anatomy
The oculomotor nerve arises at the ventral aspect of the midbrain in the interpeduncular cistern. Within the cisternal segment, the oculomotor nerves course anteriorly and laterally in the interpeduncular cistern. The nerve passes between the posterior cerebral artery (PCA) superiorly and the superiorly cerebellar artery (SCA) inferiorly ( Fig. 5 A). The nerve then passes medial to the uncus of the temporal lobes (see Fig. 5 B). The TZ of CN III.c is located, on average, approximately 1.9 mm from the apparent origin (range 1.0–4.0 mm).
CN III.c: Selected Pathologic Conditions
As the parasympathetic fibers of the nerve pass within its periphery, uncal herniation with CN III.c impingement will result in pupillary dilation caused by unopposed action of the sympathetic nervous system. Additionally, herniation of the uncus medially across the free edge of the tentorium may impinge on the nerve. Oculomotor palsy may result from compression of CN III.c by tumors or neurovascular compression, with findings on CISS correlating well with those at surgery. Isolated reversible enhancement and thickening of CN III.c has been reported in ophthalmoplegic migraine by standard imaging ; one recent review of ophthalmoplegic migraine found that nerve thickening and enhancement is seen in 75% of cases diagnosed clinically.
CN III.d (Dural Cave): Anatomy
The dural cave of the CN III is a segment of variable length and is given the name of the oculomotor cistern. The length of the oculomotor cistern is reported on anatomic studies as ranging from 3.0 to 11.0 mm, averaging 6.5 mm, and on MR imaging averaging 4.2 mm with a range of ± 3.2 mm, visualized in 75% of screening MR imaging with CISS. CN III.d (see Fig. 5 ; Fig. 6 A) extends from the oculomotor porus at the posterior margin of the oculomotor cistern to exit anteriorly into the lateral wall of the cavernous sinus at its superior extent (see Fig. 6 B).