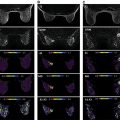
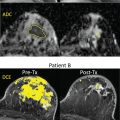
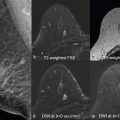
Diffusion-weighted imaging (DWI) is a promising approach for breast magnetic resonance imaging (MRI), both as a way to complement or to replace contrast-enhanced MRI. DWI measures diffusive motion, which is quantified by diffusivity, D , often measured in mm 2 /s. Assuming a Gaussian diffusion model, the measured parameter is called the apparent diffusion coefficient (ADC). DWI uses diffusion gradients to selectively attenuate the signal of water molecules that have greater diffusive motion, and this attenuation is expressed as e − bD , where b is the “ b value” of the sequence, typically in s/mm 2 , and indicates the sensitivity of the sequence to diffusion. DWI is useful in the detection of cancers because cancerous tissue often has more restricted diffusion than normal tissue and therefore appears brighter. Numerous groups have explored DWI in the context of breast cancer, with very promising results for detection, diagnosis, and assessment of treatment response. These studies are summarized in several meta-analyses and in other chapters of this book. Although DWI remains predominantly a research approach in the context of routine breast MRI, continuous improvements are enabling more robust acquisition of images with greater sensitivity to small lesions.
Breast MRI demands high spatial resolution in order to depict small lesions or to show shape, border, and heterogeneity features. Sufficient contrast-to-noise ratio (CNR) is necessary, particularly to separate potentially malignant lesions from benign lesions or normal tissue. To achieve contrast with DWI requires moderate to high b values (600–800 s/mm 2 or higher) to create contrast based on typical diffusivities (fibroglandular tissue around 1.6 × 10 –3 mm 2 /s and cancers approximately 1.0 × 10 −3 mm 2 /s), but this attenuation costs signal-to-noise ratio (SNR), and typically increasing the b value requires greater averaging to recover this SNR. Additionally, due to widely varying breast tissue composition, size, and shape, robust fat saturation is critical, especially in DWI where fat both obscures other signal due to its limited diffusion and may cause artifacts due to its strong signal and chemical shift frequency. High quality radiofrequency (RF) receive coil arrays with consistent patient placement are also important, both for adequate SNR and parallel imaging performance, and again present challenges based on the variation in patient size and breast size and shape. Although scan times are not as critical as in dynamic contrast-enhanced (DCE) imaging, long scans can result in greater patient motion, so imaging efficiency is an important consideration. Furthermore, shorter scans enable greater flexibility to use more advanced diffusion models requiring a higher number of b values and directions. Breast MRI also requires a moderately large field of view (FOV), which means gradient nonlinearities and eddy-current effects can degrade images. Finally, motion and B0 changes due to cardiac and respiratory motion can affect breast MRI. Typically, these challenges are exacerbated in DWI.
This chapter describes many of the acquisition techniques used for DWI in the breast. Fat suppressed single-shot echo-planar imaging (ss-EPI) has been the standard for breast DWI, as it is widely available and overcomes the dominant challenge of motion during DWI; however, it has limited resolution, as well as considerable distortion that results from off-resonance effects that are common in the breast. Techniques including parallel imaging, reduced FOV, and multishot EPI have been demonstrated to overcome some of these challenges. Specifically, multishot imaging variations include readout-segmented EPI (rs-EPI), multiplexed sensitivity encoding (MUSE) and PROPELLER. Other methods have also been explored in research including self-navigated spiral (SNAILS), spatiotemporal encoding (SPEN), and steady-state diffusion. All of these methods typically trade-off between robustness to off-resonance and motion, simplicity, and capability to perform quantitative DWI. These DWI acquisition approaches are generally adaptable to numerous quantitative diffusion approaches, such as diffusion-tensor imaging (DTI), intravoxel incoherent motion (IVIM), diffusion kurtosis imaging (DKI), or restriction spectrum imaging (RSI) (see Chapters 8 and 9 ).
Standard Echo-Planar Diffusion-Weighted Imaging
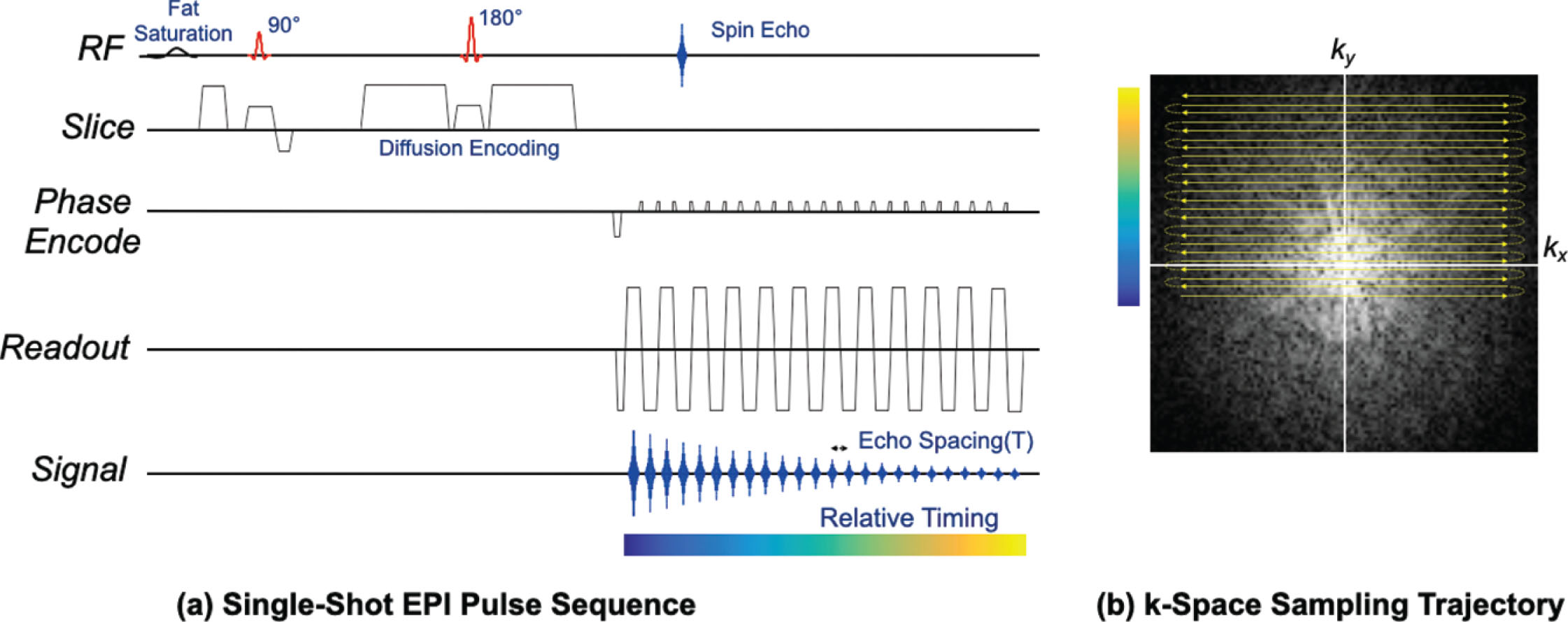
A conventional DWI acquisition in the body consists of fat suppression followed by a diffusion encoded spin-echo sequence using a single-shot EPI imaging readout, as shown in Fig. 12.1 . The large diffusion-encoding gradients have the adverse effect of making the sequence very sensitive to small bulk motion, which induces unpredictable signal phase variations across the image. The single-shot EPI readout is used to rapidly acquire the entire image after a single excitation so that such phase effects can be ignored. Furthermore, single-shot EPI avoids respiratory, cardiac, and random patient motion artifacts resulting from motion between shots. Single-shot EPI typically uses a partial-Fourier approach so that the central k-space signal is acquired before substantial T2* signal decay, equivalently reducing the echo time (TE). System calibrations and postprocessing correct for Nyquist ghosting and shading artifacts that can result from imperfections between positive and negative (rightward and leftward) readout lines. Multiple averages are often obtained to improve overall SNR, and typically the number of averages is increased for higher b values.
EPI Parameter selection
Single-shot EPI is one of the fastest approaches used for image acquisition in MRI, offering images in well under 100 ms and often much faster. Typically, the EPI trajectory is designed to maximize imaging speed. The readout gradient amplitude is maximized given limits on gradient amplitude and sample spacing (1/FOV x ). In many cases, sampling occurs on both the readout ramps and plateaus that are shown in Fig. 12.1 , with the readout gradient duration sufficient to achieve the desired k x extent and spatial resolution in x . Multiple repeated readout lobes enable sampling of more k-space lines in both directions, which maximizes speed. A phase-encode gradient initially sets the starting k y location, while “blip” gradients between each readout lobe skip by 1/FOV between k y to satisfy Nyquist sampling. The number of k y lines provides sufficient extent in k y to achieve desired phase-encode resolution. The alternating sampling typically results in ghosts, known as “FOV/2” ghosts or Nyquist ghosts, due to delays or phase differences between the readout gradients of opposite polarity induced by eddy currents or other imperfections. Typically, a reference scan is built into protocols, and enables very good correction of these ghosts, by measuring any phase and delay variation between all readout lines and correcting data for these before Fourier reconstruction.
Limitations of Epi: Readout Duration, Distortion, and Blurring
Single-shot EPI acquires all data for a slice following one excitation. The primary limitations of this approach are that the signal changes in both magnitude and phase during the acquisition. The magnitude change is due to T2* decay and leads to a loss of resolution or blurring in the phase-encode direction. Often half-Fourier acquisitions are used to sample k y from near the center out to the edge. This reduces the EPI duration and the amount of signal decay, at a cost of minor blurring from the partial-k-space reconstruction. The resulting shortened TE also reduces the inherent T2-weighted contrast that leads to T2 “shine-through” effects. Any off-resonance, caused by B0 inhomogeneity or chemical shift, causes linear phase accrual between k y lines that depends on the echo spacing (T esp ) . B0 inhomogeneity leads to spatially varying distortion, especially around the nipple, whereas the chemical shift frequency difference between water and fat causes a constant displacement of the fat with respect to water signal. Neither the off-resonance-induced distortion nor the fat/water displacement is affected by the use of half-Fourier sampling, because the k y velocity described in the next section is unchanged. Phase and amplitude differences along the readout (k x ) are smaller and usually can be ignored.
Fov Relationship
To better understand the distortion effects, it is useful to point out the fundamental relationship between displacement, the off-resonance effects, and the FOV. To support a given FOV in the phase-encode direction (FOV y ) requires a k-space line separation of ∆ k y = 1 / FOV y . If the echo spacing is T esp , the k y velocity is ∆ k y /T esp = 1 / (FOV y T esp ), and the displacement ∆ y as a function of off-resonance ∆ f is ∆ y = FOV y ∆ fT esp . This is a fundamental relationship with EPI, that off-resonance-induced distortion is proportional to the frequency offset FOV y and echo spacing T esp .
For typical parameters of T esp = 1 ms, FOV = 20 cm, and a 50-Hz frequency offset, the displacement is 1 cm. At 1.5 T and 3 T, respectively, the fat/water displacement is approximately 4.4 and 8.8 cm, which suggests the importance of fat suppression. Methods to reduce this displacement must reduce FOV y or T esp and are one of the main advances in breast DWI, as discussed later. Note that many of the approaches to reduce distortion will also reduce blurring, as they tend to shorten the readout duration and thus the amount of T2* decay.
Distortion Correction
Image distortion in EPI results primarily from B0 field variations from sources such as air-tissue boundaries or metallic biopsy markers. When the field variation is sufficiently smooth and measurable, the resulting distortion can be corrected by an image “warping” that remaps signal to original pixel locations. More rapid changes in B0 lead to “pile-up” effects, where the signal from multiple locations is mapped to one voxel, and require more advanced correction. Currently a common approach is to repeat the EPI acquisition with the phase encode gradients flipped in reverse polarity directions. Either using the data itself or in combination with a field map, the distortions can then be corrected, and the repeated acquisitions can recover more signal than a single acquisition with a field map-based correction, due to averaging. Distortion correction is particularly important when pixel-wise comparisons are desired between different sequences (e.g., to calculate the ADC maps).
Navigator Acquisitions
Navigator acquisitions are a general approach to acquire additional data to measure and possibly correct for motion during a scan. The large area of diffusion-encoding gradients, on the order of tens of degrees of rotation per micron, means that bulk motion of a few microns between the two gradients can impart substantial phase. In multishot imaging, this phase will modulate the k-space data resulting in different artifacts depending on the order in which k-space lines are acquired. Even in single-shot imaging, if a complex average of images is used (to reduce noise bias), the phase variations must be addressed. One approach to this is to add a second spin echo to the sequence and acquire a low-resolution, full-FOV image that ideally has the same motion-induced phase as the data acquired on the first spin echo. The phase of the navigator can then be used to reduce or remove phase variations in the data from the first spin echo of different excitations so that the overall image artifact is reduced or removed. Navigators have been used extensively for different multishot EPI approaches (described shortly) and for non-EPI approaches. Although they can lengthen the scan time per slice, resulting in fewer interleaved slices, they can additionally offer some correction for respiratory motion and other motion that can degrade images.
Summary
Fat-suppressed single-shot EPI is very commonly used for DWI, because the rapid readout avoids motion artifacts that would result in multishot techniques, and most importantly the bulk motion–induced phase can usually be ignored. This sequence is widely available on most MRI systems, with careful corrections built in. The main limitations of distortion and blurring have been studied extensively and are described in detail in the remainder of this chapter. Specifically for breast MRI, the choice of fat suppression is important, and different approaches are described next.
Fat-Suppression Methods
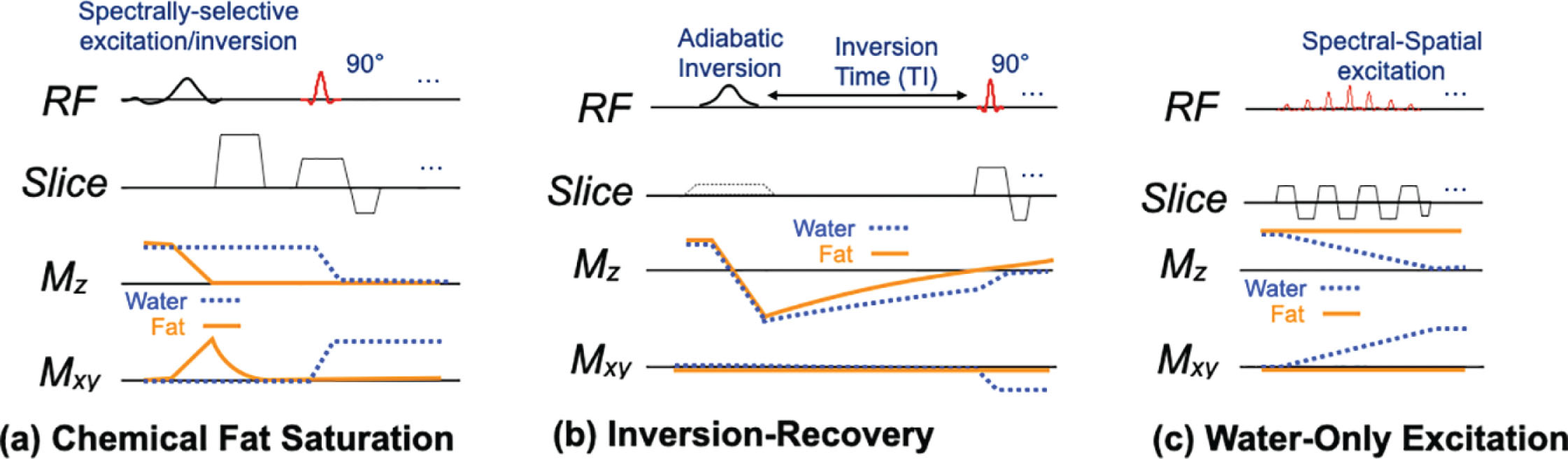
Fat suppression is typically based on frequency differences or T1 differences between fat and water. Chemically selective saturation (CHESS) or inversion pulses precede the sequence. Fat suppression is important to avoid the artifacts that can result from fat, particularly the chemical shift displacement in EPI that often results in ghosting, especially when parallel imaging is used. In addition, fat has minimal diffusivity and so appears very bright on DWI, which obscures other tissue features and reduces the dynamic range of these features. Fat-suppression approaches may loosely include reducing the signal from fat or separating the water and fat signal, typically done with Dixon-based methods. In DWI in the breast, the former group of approaches is typically used, making use of either T1 differences (STIR) or the chemical shift frequency difference between fat and other tissues, with the schemes shown in Fig. 12.2 .
Chemically Selective Fat Suppression
Chemically selective fat suppression, often simply “fat saturation,” uses a spectrally selective pulse to selectively excite only the fat signal, as shown at the start of the sequence in Fig. 12.1 . An applied gradient then dephases the fat spins so that they contribute no signal. The standard excitation and imaging follow the fat saturation block, with timing unaffected by the fat saturation. Fat saturation is simple and fast but can suffer from sensitivity to B0 and B1 variations. In the breast, susceptibility variations from proximity to the lungs or air-tissue boundaries near the chest wall, axillae, nipple, or the inferior portion of the breast often cause fat suppression to fail. This occurs when the broad spectral fat peak shifts outside the suppression bandwidth of the pulse. Fat saturation can use a simple 90-degree excitation or an inversion followed by an inversion time. Adiabatic spectral inversion recovery (e.g., ASPIR or SPAIR on different systems) is sometimes used to reduce sensitivity to off-resonance at a cost of increased RF power.
Stir
Short TI inversion recovery (STIR) suppresses fat based on the shorter T1 (typically 250 ms at 1.5 T and 350 ms at 3.0 T) of fat compared with that of other tissues (typically over 1 second) in the breast. As the name suggests, STIR uses in inversion pulse, which is usually not spatially selective, to invert all signal. After an inversion time (TI) of 150 to 180 ms, normal excitation and imaging proceeds. This time is carefully chosen to null the signal from fat (i.e., based on e − TI/T 1 ≈ 0.5). A strength of STIR is the robustness to static magnetic field inhomogeneities, and with the commonly used adiabatic inversion pulses, additional robustness to transmit B1 field variations. The main drawback of STIR is that the inversion-recovery causes a loss in signal of about 30% to 40%, which translates to a need to double the number of averages to achieve adequate SNR. Additionally, it is challenging to interleave other slices within the inversion time, so the number of slices that can be interleaved within one repetition is reduced, which reduces efficiency. Finally, in cases where DWI is performed after DCE, the presence of contrast can result in nulling of other tissue or tumor because of its shortened T1, which can be similar to that of fat.
Water-Only Excitation
Water-only excitation or “spectral-spatial” pulses can simultaneously excite a narrow frequency band as well as the imaging slice. As Fig. 12.2C shows, the pulse is a series of slice-selective pulses, with overall scaling that provides selectivity to only the water frequency. The pulse is similar in duration to chemical saturation, and the length can increase the TE (or equivalently the time between excitation and refocusing in a spin-echo sequence). Water-only pulses are typically less prone to residual fat signal than fat saturation, because the water peak (usually narrower than the fat peak) is less likely to shift outside the excitation band. Unlike fat saturation, where B1 variations cause unsuppressed fat, water-only excitations have the same B1 shading/contrast variations as any sequence. The pulse length, typically around 10 to 12 ms at 1.5 T, can be shortened to about 5 to 6 ms at 3 T.
Shimming
Regardless of technique, fat suppression is absolutely essential for breast DWI. Aside from different vendor systems and field strengths, the wide range of breast sizes and shapes combined with variations in coils and positioning makes reliable, robust fat suppression a continuous challenge. Because STIR costs time efficiency as well as SNR, chemical-shift-based approaches are most commonly used and rely on careful shimming to minimize the range of B0 variations. Numerous approaches to improving shimming have been studied, with no simple robust approach. Approaches including dual-volume shimming, “smartshim,” dynamic per-slice shimming, or even independently shimmed slab excitation over each breast have been explored, but applicability to all techniques still remains a challenge, and some shim approaches will add additional examination time. The availability of the aforementioned shimming approaches also varies with different vendor products, so empirical protocol optimization is often the best way to achieve the best results.
Summary
Fat suppression is critical for breast DWI, primarily because the fat is much brighter than other tissue owing to its short T1 relaxation, moderately long T2, and limited diffusivity, and therefore it obscures the appearance of lesions. Additionally, the chemical-shift frequency results in a displacement in EPI that causes fat to overlap other parts of the image and causes ghosting when parallel imaging is used without consideration of fat, leading to contamination of tissue diffusivity measures. Multiple approaches can be used for fat suppression, mostly independently of the image acquisition portion of the sequence, each with different trade-offs with respect to timing considerations, sensitivity to B0 and B1 variations, and SNR.
Reducing Distortion, Blurring, and Scan Times With Available Methods
Major limitations of EPI are the distortion and blurring effects that are fundamentally related to the phase-encode FOV and the moderately long scans that are required to achieve adequate slice coverage and SNR. This section explores some of the more readily available improvements to single-shot EPI. One way to mitigate distortion and blurring artifacts in EPI is to speed the k y traversal of the acquisition. This can be done either by shortening the interecho spacing T or by increasing the spacing between k y lines. Limitations on gradient amplitudes and slew rates force a reduced k x traversal extent if T is to be reduced; this is explored later in the section on rs-EPI (rs-EPI). Alternatively, increasing ∆ k y results in a reduced acquisition FOV in the y direction that will result in aliasing or wrap artifact. The aliasing can be reduced either by basic approaches that limit the excited FOV along k y or by using parallel imaging. There is also considerable similarity with interleaved multishot acquisitions, which essentially combine multiple reduced-FOV images, as discussed in more detail later. Finally, simultaneous multislice (SMS) imaging offers improved scan efficiency by acquiring multiple slices together, though it does not shorten echo spacing to reduce either distortion or T2* blurring.
Choice of Geometry
The choice of imaging geometry in breast MRI involves many factors, including the goal of reducing the k y FOV. Notably, at the resolutions used for DWI, the readout duration is usually limited by the gradient slew rate and amplitude rather than by the sampling rate. This means that the additional FOV in the readout (k x ) direction does not affect the echo spacing T , and therefore ideally the largest FOV dimension could be oriented in the frequency-encode direction and smallest FOV dimension in the phase-encode direction. Artifacts such as aliasing or cardiac or respiratory motion are usually minimized in the readout direction, and often in breast MRI the readout direction is selected as anterior/posterior (A/P) so that cardiac pulsation artifact does not interfere with breast tissue. For axial bilateral breast imaging, often a rectangular FOV is acceptable, with larger coverage needed in the right/left (R/L) direction (typically 32–40 cm) than in the A/P direction. For axial DWI, frequency R/L can therefore enable a smaller FOV than frequency A/P, though the cardiac artifact should be considered. Alternatively, sagittal imaging is sometimes performed with a small FOV in the superior/inferior (S/I) direction. Overall, there are trade-offs to imaging geometry, though most clinical DWI protocols currently use an axial acquisition with readout R/L to limit aliasing from arms or readout A/P to reduce cardiac pulsation artifact. As described later, phased-array coils enable reduced FOV and parallel imaging. Because it is the reduced FOV rather than the full FOV that determines distortion and T2* blurring, these geometry considerations must be considered together with the coil geometry.
Breast Phased-Array Coils
Dedicated breast RF coils (typically receive-only) are essential for high-quality breast MRI. Generally, such coils are designed for robustness across a variety of patient sizes, aiming to provide relatively uniform signal coverage as the number of elements increases. Commercially available 8-channel coils became standard for imaging during 2001 to 2010 and are still widely used. However, 16-channel coils from numerous vendors have become commonplace both for 1.5 T and 3.0 T imaging. The smaller receive elements of higher-channel-count coils offer greater sensitivity based on higher signal as well as lower noise per channel. Additionally, when these sensitivities vary considerably from one element to another, parallel imaging can be used to further improve performance, as described next. Dedicated breast coils have limited sensitivity to signal from tissues deep in the body and from the patient’s arms (when imaged with “arms down” along their sides), so it can simplify the selection of geometry and enable reduction of the k y FOV in different directions.
Parallel Imaging
Parallel imaging was a transformational advance in MRI that uses the varying spatial sensitivity of coil arrays to reconstruct images that are acquired with a reduced FOV, which is of course synonymous with skipping k y lines. The reduced-FOV images take less time because the k y spacing is larger, and therefore there are fewer acquired k y lines. Common reconstruction approaches are to undo aliasing (sensitivity encoding, SENSE) or to fill in missing k-space lines (GRAPPA), though many variants and hybrids of these approaches exist. Standard parallel imaging is applied only along a phase-encode dimension. A simplistic but intuitive example of breast parallel imaging is that if two coil elements are each sensitive to one breast, then a reduced FOV image of each breast can be simultaneously acquired, and these can be stitched together to form a bilateral image in the same scan time. Note that this example requires the phase-encode direction to be L/R. Although the coil sensitivities overlap, there are advantages to thinking of parallel imaging as “building up” FOV by using multiple coil elements.
Parallel Imaging and EPI
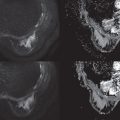
In EPI, the use of parallel imaging has strong additional benefits: the increased k y spacing increases the rate of k y traversal, which reduces both blurring and distortion. These effects are clearly demonstrated in Fig. 12.3 , which shows an example of axial DWI with the phase-encode direction R/L and three reduction factors (R = 1, 2, and 4), with reduced distortion and blurring when a greater reduction is used. These benefits arise directly from the FOV relationship described earlier. Parallel imaging must be applied in the phase-encode (k y ) direction and requires a multichannel coil with sensitivity variation in this direction. Theoretically, the reduction factor R should not exceed the number of coil elements in that direction. In practice, in breast imaging, 8-channel coils are typically capable of R = 2 (L/R), and 16-channel coils can achieve R = 4 L/R and R = 2 S/I or A/P. However, the key question for distortion and blurring is the reduced phase-encode FOV (phase-encode FOV/R), not only the reduction factor R. For example, it may be possible to use a smaller FOV to cover A/P and use the readout direction to cover the full L/R FOV with better overall performance in spite of a lower R. Another form of parallel imaging, SMS imaging, uses coil sensitivities to speed up acquisition by acquiring multiple slices at the same time. SMS, which is described shortly, generally does not change distortion and resolution in EPI.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree