The most common sites of nerve entrapment are in the upper extremity, commonly diagnosed based on clinical findings and electrophysiologic studies. Cross-sectional imaging modalities, such as ultrasonography and magnetic resonance (MR) imaging, have been used to enhance diagnostic accuracy and provide anatomic mapping of abnormalities. MR neurography offers multiplanar high-resolution imaging of upper extremity nerves as well as adjacent soft tissues, and provides an objective assessment of the neuromuscular anatomy and related abnormalities. This article reviews the normal 3-T MR neurographic appearance of the upper extremity nerves, and abnormal findings related to injury, entrapment, and other pathologic conditions.
Key points
- •
High field strength allows better 3-dimensional (3D) imaging, therefore 3T MR scanning is preferred.
- •
The imaging around the joints is best accomplished using joint-specific coils.
- •
Auto-shimming is essential before 3D diffusion-weighted PSIF (reversed steady-state free precession) and diffusion tensor imaging to avoid ghosting and attain uniform fat suppression.
- •
Additional long-axis fluid-sensitive 2D images (fat-suppressed proton density or short-tau inversion recovery) are acquired to evaluate the joints and other structures in the imaging field of view.
- •
Nerve variations and magic angle artifact should be kept in mind to avoid overcalling neuropathy.
- •
Fascicular abnormality or nerve enlargement are definitive MRN imaging signs of neuropathy.
- •
The critical findings in grading nerve injury are detection of neuroma and nerve transection.
Introduction
Nerve entrapment and injury of the upper extremity is more common and can be more complex than that of the lower extremity, with the existence of several entrapment sites along the course of major upper limb nerves. These lesions can be diagnosed based on clinical findings and electrophysiologic studies, such as electromyography and nerve conduction studies. However, these studies are invasive, can be uncomfortable, can be falsely negative, frequently show low positive predictive values, and can be nonlocalizing in patients with mild lesions or in cases of severe axonal injury with low distal compound action potentials. Such studies are normal in cases of neurapraxia (mild nerve injury) and can be normal up to 7 to 14 days after nerve injury, even when the nerve is physically divided. Ultrasonography has been widely used for common sites of entrapment, such as carpal tunnel or cubital tunnel syndromes, because of its low cost, portability, and real-time capability. However, ultrasonographic study requires operator skill, may be limited by local calcifications/dense scarring, does not demonstrate nerve and muscle signal intensity alterations as with magnetic resonance (MR) imaging, and frequently lacks objectivity. Therefore there has been much interest in development and use of high-resolution MR imaging techniques capable of more precisely delineating nerve abnormality and its underlying cause. High-resolution MR imaging that provides multiplanar isotropic depiction of the nerves using a combination of 2-dimensional (2D) and 3-dimensional (3D) imaging with and without uniform fat-suppression techniques, also referred to as MR neurography, has been increasingly used for peripheral nerve evaluation. MR neurography not only reveals the morphologic characteristics of nerves but also provides information on pathologic processes including nerve inflammation, edema, fibrosis, and fat proliferation. It has been increasingly used in the assessment of lesions affecting peripheral nerves, plexus, and spinal nerve roots. This article reviews the normal 3-T MR neurographic appearance of the upper extremity nerves, and abnormal findings related to injury, entrapment, and other pathologic conditions.
Introduction
Nerve entrapment and injury of the upper extremity is more common and can be more complex than that of the lower extremity, with the existence of several entrapment sites along the course of major upper limb nerves. These lesions can be diagnosed based on clinical findings and electrophysiologic studies, such as electromyography and nerve conduction studies. However, these studies are invasive, can be uncomfortable, can be falsely negative, frequently show low positive predictive values, and can be nonlocalizing in patients with mild lesions or in cases of severe axonal injury with low distal compound action potentials. Such studies are normal in cases of neurapraxia (mild nerve injury) and can be normal up to 7 to 14 days after nerve injury, even when the nerve is physically divided. Ultrasonography has been widely used for common sites of entrapment, such as carpal tunnel or cubital tunnel syndromes, because of its low cost, portability, and real-time capability. However, ultrasonographic study requires operator skill, may be limited by local calcifications/dense scarring, does not demonstrate nerve and muscle signal intensity alterations as with magnetic resonance (MR) imaging, and frequently lacks objectivity. Therefore there has been much interest in development and use of high-resolution MR imaging techniques capable of more precisely delineating nerve abnormality and its underlying cause. High-resolution MR imaging that provides multiplanar isotropic depiction of the nerves using a combination of 2-dimensional (2D) and 3-dimensional (3D) imaging with and without uniform fat-suppression techniques, also referred to as MR neurography, has been increasingly used for peripheral nerve evaluation. MR neurography not only reveals the morphologic characteristics of nerves but also provides information on pathologic processes including nerve inflammation, edema, fibrosis, and fat proliferation. It has been increasingly used in the assessment of lesions affecting peripheral nerves, plexus, and spinal nerve roots. This article reviews the normal 3-T MR neurographic appearance of the upper extremity nerves, and abnormal findings related to injury, entrapment, and other pathologic conditions.
Technical considerations in MR neurography
Technical considerations in MR neurography are extensively discussed in another article in this issue by Chhabra and colleagues. In brief, some key points should be followed in upper extremity imaging. Higher field strength allows better 3D imaging, therefore 3-T scanning is preferred. The imaging around the joints is best accomplished using joint-specific coils. Contiguous imaging, for example elbow and forearm, should be performed using separate fields of view for elbow and forearm, ideally with separate phased array coils for each. To avoid wraparound artifacts, dead (air) space around the extremity should be avoided or minimized as much as possible to attain the highest possible resolution and contrast. 2D imaging using axial T1-weighted and axial T2 spectral adiabatic inversion recovery (SPAIR) imaging is performed with less thickness than the plexus or lower leg imaging, to keep in-plane resolution between 0.3 and 0.4 mm. The slice thickness is kept at 4 mm, 3 mm, and 2 mm for upper arm, forearm, and wrist, respectively. 3D diffusion-weighted (DW) reversed steady-state free precession (PSIF) imaging is very useful for displaying the anatomy along the long axis of the extremity. 3D SPAIR sampling perfection with optimized contrasts using varying flip-angle evolutions (SPACE) works better in extremities than 3D short-tau inversion recovery (STIR) SPACE, because of its higher signal-to-noise ratio. Auto-shimming is essential before 3D DW PSIF and diffusion tensor (DT) imaging, to avoid ghosting and attain uniform fat suppression. Additional long-axis fluid-sensitive 2D images (fat-suppressed proton density or STIR) are acquired to evaluate the joints and other structures in the imaging field of view.
Brachial plexus branches
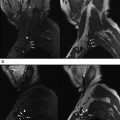
The brachial plexus has a complex anatomy, and is formed by the contribution of the ventral branches coming from the 4 lower cervical and first thoracic nerves; it is covered elsewhere in this issue by Lutz and colleagues. The discussion here focuses on brachial plexus branch nerves relevant to the upper extremity. Whereas certain nerves come directly from the plexus, such as the spinal accessory nerve, the most commonly imaged nerves include the 3 peripheral nerves of the upper limb: the median nerve, ulnar nerve, and radial nerve. The radial nerve arises from the posterior cord; the majority of the median nerve arises from the lateral cord while the median nerve and the ulnar nerve receive contributions from the medial cord. It is worth mentioning that brachial plexus abnormality can extend into the peripheral nerves ( Fig. 1 ). Proximal plexus abnormality can also cause double-crush syndrome and present with distal peripheral nerve symptomatology at one of the entrapment sites, and this syndrome should be considered in the differential diagnosis, both clinically and during imaging for proper management. For example, thoracic outlet syndrome causing C8 and/or T1 neuropathy may predispose the ulnar nerve to entrapment distally or exacerbate ulnar neuropathy symptoms.
Ulnar nerve
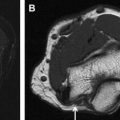
The ulnar nerve originates from the medial cord of the brachial plexus (C8, T1) with occasional contributions from the C7 nerve root. It courses along the posteromedial compartment of the upper arm in a relatively straight fashion. At the mid-arm level, the nerve penetrates the medial intermuscular septum and courses adjacent to the epimysium of the medial head of the triceps and deep fascia before reaching the cubital tunnel, where it passes between the medial epicondyle of the humerus and the olecranon at the condylar groove. Here, it lies deep to the cubital tunnel retinaculum (CTR), also known as the Osborne fascia, and aponeurosis formed between the 2 heads of the flexor carpi ulnaris. The nerve courses straight between superficial and deep compartments of the forearm along its medial side and at the wrist, and passes through a fibro-osseous tunnel known as the Guyon canal ( Fig. 2 ). The floor of this tunnel is formed by hamate, hypothenar muscles, and flexor retinaculum; the roof is composed of the pisohamate ligament, volar carpal ligament (the terminal thickened portion of the antebrachial fascia), palmaris brevis muscle, and fibers of the palmar fascia. There is typical topographic arrangement of dorsal cutaneous sensory, motor, and sensory fascicles within the nerve in the forearm from medial to lateral. The ulnar nerve enters the Guyon canal after separating off its dorsal cutaneous branch, and splits into the superficial sensory nerve and the deep motor nerve during its course within the canal. The 2 terminal branches of the nerve can be easily visualized: the sensory branch running in proximity to the ulnar artery; and the motor branch, which courses more deeply, adjacent to the medial surface of the hamate hook. The ulnar nerve may be damaged at any place along its course in the upper extremity. In addition, the aforementioned double-crush phenomenon should be considered in cases of ulnar neuropathy.
Cubital Tunnel Syndrome
Ulnar nerve entrapment (UNE) at the elbow is the second most common entrapment neuropathy. Cubital tunnel syndrome is defined by ulnar neuropathy beneath or near the CTR. Various hypotheses of ulnar neuropathy at this site have been hypothesized. Although evidence is limited, it seems that repeated elbow flexion, which can cause a 4- to 5-fold increase of pressure inside the cubital tunnel, and repeated external pressure on the ulnar nerve from chronically resting the elbow on firm surfaces provide possible explanations for neuropathy. Early stages of peripheral nerve entrapment may be associated with intraneural vascular congestion, endoneurial edema, and hyperemia. Over time it leads to initiation of intraneural fibrosis, focal demyelination, and axonal loss. Other causes of cubital tunnel narrowing include a thickened CTR/Osborne fascia, an anomalous anconeus epitrochlearis muscle, and a range of space-occupying lesions (eg, tumors, scarring, heterotopic ossification, loose bodies, fracture fragments, and ganglion cysts). The clinical features of UNE are heterogeneous, and include intermittent paresthesias and sensory loss in the ulnar nerve distribution, especially the ring or/and small fingers, with or without atrophy; and weakness of the ulnar nerve innervated hypothenar and interosseus muscles and pain, which can be limited to over the cubital tunnel or radiate to the shoulder or wrist. The Tinel sign over the cubital tunnel is frequently present.
Imaging Findings
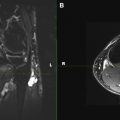
Electrophysiologic studies are usually used to confirm the diagnosis of UNE, but these studies may be nonlocalizing in patients with mild lesions or in those with severe axonal injury and low amplitudes of distal compound muscle action potential. MR neurography imaging findings reveal abnormal T2 hyperintensity of the nerve approaching adjacent in-plane veins ( Fig. 3 ). Mild hyperintensity of the nerve is commonly seen as an incidental finding, similar to slowing of nerve conduction velocity on electrophysiologic study, and it should be reported as a nonspecific finding to be correlated clinically for neuropathy. Nerve enlargement and fascicular abnormality, with or without distal muscle denervation change, are seen with moderate neuropathy. Abrupt signal change in the nerve from bright-black-bright indicates severe constriction or nerve injury, and correlates with severe neuropathy. Severe entrapment is usually seen with organic mass lesions or with reentrapment in previously anteriorly transposed nerve ( Fig. 4 ). The latter may also show abnormal angulation along the course of the nerve caused by kinking by scarring, along with regional muscle denervation edema in the flexor carpi ulnaris, flexor digitorum profundus of fourth and fifth digits, and interosseous and hypothenar muscles of the hand. Indeed, muscle wasting is more prevalent among patients with cubital tunnel syndrome than in patients with carpal tunnel syndrome. DT imaging selectively shows abnormally hyperintense nerve on the tensor image, with low fractional anisotropy values owing to demyelination and/or axonal degeneration. In addition, MR neurography is useful for the assessment of patients at risk for double-crush injuries, because MR neurography shows the most abnormal findings at and immediately proximal to the site of injury. Although ultrasonography is better for real-time assessment of ulnar nerve subluxation or dislocation, MR neurography can show secondary findings of ulnar nerve abnormalities, a thickened or absent retinaculum, or a space-occupying lesion, such as an accessory muscle or low muscular insertion of the medial head of the triceps ( Fig. 5 ). Surgical release of an entrapped nerve is commonly accompanied by subcutaneous or submuscular transposition anteriorly. Transposed but otherwise normal nerve shows mild increased signal on MR neurography that decreases over time. Reentrapment of the anteriorly transposed ulnar nerve may occur because of overzealous dissection and development of perineural fibrosis, necessitating repeat surgical ulnar nerve release. Findings of MR neurography in symptomatic patients include 1 or a combination of findings, such as persistent/increased T2 signal abnormality approaching or more than the adjacent in-plane venous signal intensity, expanded nerve fascicles in the enlarged hyperemic ulnar nerve with or without abnormal angulations caused by perineural fibrosis, and/or hemorrhage. Finally, long-segment nerve abnormality on MR neurography, or ulnar neuropathy in the proximal arm or distal arm away from the site of entrapment and/or multiple nerve lesions, indicates a systemic cause of neuropathy: lymphoma, neurofibromatosis, or an inflammatory condition such as multifocal motor neuropathy ( Figs. 6 and 7 ).
Guyon Canal Syndrome
The Guyon canal is another common potential site of ulnar nerve compression. The location of compression may be classified among 3 zonal locations :
Zone 1: Proximal edge of volar carpal ligament to bifurcation of ulnar nerve, where patients present with combined motor and sensory deficits
Zone 2: From nerve bifurcation to just distal to fibrous arch of hypothenar muscles, where patients may present with pure motor deficit
Zone 3: Distal end of the canal containing superficial sensory branch, where patients may present with pure sensory deficit
Nerve entrapment in zones 1 and 3 are the most common, although most patients have symptoms in more than 1 zone. Entrapment neuropathy can result from extrinsic compression factors (eg, bicycle riding, judo, tennis), intrinsic space-occupying lesions (ganglion, lipoma, ulnar artery aneurysm, uremic tumoral calcinosis, trauma, and anomalous muscle, such as accessory abductor digiti minimi), or from chronic traction neuropathy caused by regional fracture or use of crutches.
Imaging Findings
The value of MR imaging in Guyon canal syndrome is in the detection of space-occupying lesions within the canal and/or signal intensity and size changes of the nerve itself. Secondary signs of neuropathy include denervation atrophy/edema of the hypothenar muscles, third/fourth lumbricals, and interossei muscles. Isolated deep-bundle neuropathy may also be diagnosed on high-resolution 3-T MR neurography, which is a difficult diagnosis on electrodiagnostic testing ( Fig. 8 ).
Radial nerve
The radial nerve is the terminal branch of the posterior cord of the brachial plexus (C5–C8, T1). In the arm, it courses posterior to the humerus in the spiral groove and penetrates the lateral intermuscular septum to exit the spiral groove and lie anterior to the lateral condyle beneath the brachioradialis muscle. The radial nerve innervates the triceps muscle, brachioradialis muscle, anconeus muscle, and inferolateral half of the brachialis muscle. At the level of the elbow joint, the radial nerve splits into a superficial sensory branch that courses in the forearm along the brachioradialis muscle, and a deep motor branch that traverses deep to the arcade of Frohse to penetrate the supinator muscle. The radial tunnel refers to an enclosed space from the level of the radiocapitellar joint to the point of the proximal part of the supinator muscle. It is bounded by the extensor carpi radialis longus and brevis muscles (anterolaterally), brachialis muscle (medially), capitellum (posteriorly), and the supinator muscle (anteriorly). Its contents include the radial nerve, which divides in the proximal forearm into superficial (primarily sensory) and deep (primarily motor) branches. The superficial branch of the radial nerve descends in the forearm under the brachioradialis and eventually pierces the deep fascia near the back of the wrist (see Fig. 2 ). The deep branch of the radial nerve pierces the supinator muscle, after which it is known as the posterior interosseous nerve (PIN). At the elbow, the deep branch innervates the extensor carpi radialis brevis muscle and the supinator muscle. The PIN innervates the posterior-compartment muscles including the extensor carpi ulnaris, abductor pollicis longus, extensor digitorum communis, extensor pollicis longus and brevis, extensor indicis proprius, and extensor digiti minimi. The radial sensory nerve courses into the dorsum of the hand and provides cutaneous innervation to the proximal parts of the dorsum of the thumb, index finger, and middle finger.
Radial neuropathy may result from brachial plexopathy, proximal nerve injury in the axilla from crutches or shoulder dislocation, a manifestation of intraneural or extraneural mass lesion, or its related surgery (see Fig. 1 ; Figs. 9–11 ). Compression sites from proximal to distal include thickened lateral intramuscular septum and tight sheath of lateral head of triceps, fibrous bands from the radiocapitellar joint, the tendinous edge of the extensor carpi radialis brevis muscle, prominent radial recurrent vessels, or thickened arcade of Frohse (tendinous proximal edge of supinator). Entrapment of the PIN beneath the thickened arcade of Frohse is the most commonly reported lesion, which may develop after repetitive pronation-supination microtrauma.