Intracranial vessel wall imaging (IVWI) is an advanced MR imaging technique that allows for direct visualization of the walls of intracranial blood vessels and detection of subtle pathologic vessel wall changes before they become apparent on conventional luminal imaging. When performed correctly, IVWI can increase diagnostic confidence, aid in the differentiation of intracranial vasculopathies, and assist in patient risk stratification and prognostication. This review covers the essential technical underpinnings of IVWI and presents emerging clinical research highlighting its utility for the evaluation of multiple intracranial vascular pathologies.
Key points
- •
High-resolution magnetic resonance intracranial vessel wall imaging (IVWI) provides valuable insights into specific pathologic processes affecting the walls of intracranial blood vessels.
- •
IVWI allows for differentiation of intracranial vasculopathies that would not otherwise be possible using conventional luminal imaging techniques.
- •
When used appropriately, IVWI boosts diagnostic confidence and may aid in patient prognostication.
Introduction
Vessel wall imaging is an advanced MR imaging technique that allows for direct visualization of the walls of intracranial and extracranial blood vessels and can be used to evaluate numerous vascular pathologies. Intracranial vessel wall imaging (IVWI) serves a complementary role to conventional luminal imaging and should be performed in conjunction with such techniques. Unlike luminal imaging techniques that assess changes in vessel contour and caliber, IVWI allows for direct evaluation of the vessel wall and provides valuable diagnostic insights into vessel wall pathology. Among its many applications, IVWI has been shown to reliably distinguish between different intracranial vasculopathies, boosting diagnostic confidence and adding clinical value to the imaging workup.
Historically, vessel wall imaging techniques were used to evaluate the major cervical arteries for vessel wall pathology, with specific emphasis on characterization of vulnerable carotid plaques. Because of the unique anatomic features of the intracranial vasculature and the different environment within which they are found, extracranial vessel wall imaging techniques differ from intracranial applications. Unlike the major cervical arteries, intracranial arteries are smaller in size, possess highly tortuous orientations, and are commonly surrounded by fluid intensity, all of which pose unique technical challenges for IVWI.
Considering these challenges, IVWI techniques must possess high spatial resolutions with high signal-to-noise and contrast-to-noise ratios (SNR and CNR, respectively) to be of diagnostic quality. Specialized blood and cerebrospinal fluid (CSF) suppression techniques can be performed as part of any diagnostic IVWI protocol. Given both the increasing role IVWI plays in the evaluation of intracranial vascular pathology and the rapidly evolving technical developments associated with this advanced imaging technique, the purpose of this article was to review the technical underpinnings of current IVWI techniques and present evidence supporting their use in the workup and management of clinically important intracranial vascular pathologies.
Technical foundations of intracranial vessel wall imaging
At our institution, we rely heavily on 3-dimensional (3D) IVWI techniques for vessel wall visualization. Three-dimensional IVWI sequences have high in-plane and through-plane spatial resolutions with isotropic spatial resolutions of 0.4 to 0.7 mm 4 . Unlike 2D sequences, isotropic 3D acquisitions allow for customizable reconstructions perpendicular to almost any local vessel orientation, with minimal volume-averaging artifacts, allowing for improved visualization of the vessel wall. , Although 3D techniques take more time per acquisition to acquire than 2D methods, with multiplanar reconstructions, overall scan time is reduced.
Blood and Cerebrospinal Fluid Suppression Techniques
Most 3D IVWI techniques rely on 3D variable refocusing flip angle (VRFA) turbo spin echo (TSE)/fast spin echo (FSE) sequences because of their intrinsic black blood properties achieved through a combination of intravoxel dephasing of moving blood spins and the formation of simulated echoes. Motion-sensitized–driven equilibrium (MSDE) is a technique that uses 3D flow-dephasing gradients applied before TSE/FSE sequences to further suppress luminal flow signal. , Although MSDE can improve blood suppression, this technique leads to overall signal loss and T2 decay, somewhat limiting its utility with high-resolution acquisitions such as IVWI.
CSF suppression is a vital component of diagnostic IVWI. Delayed alternating with nutation for tailored excitation (DANTE) is a technique used for IVWI that allows for both CSF and blood suppression through a series of low flip angle nonselective pulses interleaved with gradient pulses with short repetition times. Unlike other techniques, DANTE has no effect on tissue contrast, covers a large volume, and minimizes artifacts from turbulent or slow flow but may require slightly longer acquisition times. DANTE also provides strong blood suppression, improving nulling of postcontrast blood signal, that will have shortened T1 signal relative to the precontrast acquisition. Alternatively, Anti-Driven-Equilibrium (ADE; Philips Health, Best, Netherlands) or Restore (Siemens Healthineers, Erlangen, Germany) pulse sequences use a positive 90° pulse at the end of an echo train to tip transverse magnetization into the negative longitudinal plane, effectively suppressing transverse magnetization and minimizing CSF signal. Sequences incorporating ADE/Restore provide strong CSF suppression. Table 1 details the IVWI protocol used at our institution. We rely on a DANTE preparatory pulse for improved blood and CSF suppression on postcontrast imaging, with Restore for CSF suppression on precontrast and postcontrast acquisitions. All sequences are acquired on a 3T scanner with use of a 64-channel neurovascular coil.
| Scan Parameter | 3D Restore-T1W SPACE Pre- & Postcontrast | 3D DANTE T1W SPACE Postcontrast | 3D TOF MRA |
|---|---|---|---|
| TR/TE, ms | 900/15 | 900/15 | 22/3.69 |
| In-plane resolution, mm | 0.56 × 0.56 | 0.56 × 0.56 | 0.3 × 0.3 |
| Slice thickness, mm | 0.56 | 0.56 | 0.5 |
| Flip Angle (°) | Alternating | Alternating | 18 |
| Field of view, mm 3 | 180 × 180 | 180 × 180 | 205 × 184 |
| Matrix | 320 × 320 | 320 × 320 | 384 × 384 |
| GRAPPA | 2 | 2 | 2 |
| Averages | 1 | 1 | 1 |
| Restore | On | On | – |
| Scan time a | 8:08 | 8:38 | 5:40 |
Intracranial atherosclerotic disease
Intracranial atherosclerotic disease (ICAD) is characterized by abnormal fibrotic tissue deposition within the vessel wall intermixed with varying amounts of internal lipid, cellular debris, and hemorrhage. Although lesions complicated by plaque ulceration and intraplaque hemorrhage (IPH) are less common than in extracranial atherosclerotic disease, they remain strongly associated with ischemic events. ICAD is strongly associated with underlying hypertension across ethnicities. , Diabetes mellitus has been shown to be an independent risk factor for the development of posterior circulation ICAD in Korean populations older than 50. , The role dyslipidemia plays in the development of ICAD is less certain. ,
The overall prevalence of symptomatic ICAD stenoses ranges from 25% to 53% depending on the study population. In work performed by Leung and colleagues, severe ICAD was present in at least 1 artery in 30% of Chinese patients in their sixth and seventh decades of life and in at least 1 artery in 50% of these individuals in their eighth and ninth decades of life. Asymptomatic lesions are also of clinical significance, as the WASID Trial demonstrated that the ischemic stroke risk in vascular territories downstream from asymptomatic ICAD plaques was approximately 3.5% per year.
Intracranial Atherosclerotic Disease Intracranial Vessel Wall Imaging Findings
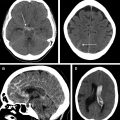
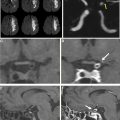
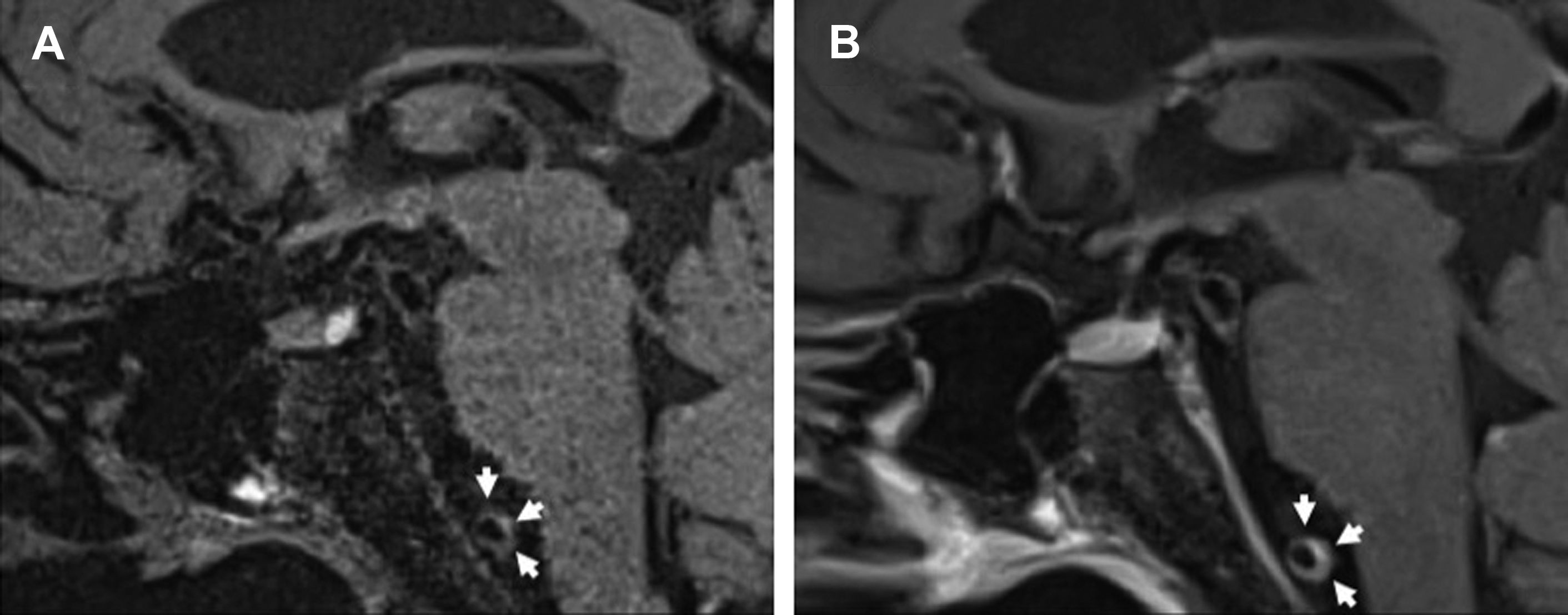
On IVWI, ICAD commonly produces focal eccentric vessel wall thickening with concentric wall involvement being less common. , Although luminal imaging (computed tomography angiography, magnetic resonance angiography, digital subtraction angiography) is the reference standard for ICAD evaluation, stenosis assessment frequently underestimates the presence and burden of ICAD. This is because ICAD frequently outwardly remodels, and can reach significant burden before resulting in appreciable luminal stenosis ( Fig. 1 ). In the evaluation of 339 autopsy cases with ischemic stroke, 40% of ICAD lesions found showed minimal to no stenosis. Interestingly, positive wall remodeling has been associated with embolic events and ischemic stroke. ICAD plaques commonly demonstrate T2 hyperintensity, with juxtaluminal T2 hyperintensity shown histologically to represent fibrous cap. Plaques frequently enhance (see Fig. 1 ) to variable degrees, but avid plaque enhancement has a strong association with ischemic events. , Avid plaque enhancement is an independent risk factor for ischemic stroke with an adjusted odds ratio of 17.4, and is also associated with a stroke recurrence rate of 30.3% at a median follow-up of 18 months, whereas nonenhancement is associated with only a 6.8% recurrence rate. Although asymptomatic plaques can also enhance, nonenhancing plaques are typically non-culprit.
Intracranial vasculitis
Primary angiitis of the central nervous system (PACNS) affects the small to medium-sized leptomeningeal and parenchymal arteries but spares the vasculature outside of the CNS. It is a rare entity with an estimated incidence of 2.4 cases per 1,000,000 person-years. In contrast, secondary CNS vasculitides are intracranial manifestations of systemic disease and include both infectious and noninfectious etiologies. Infectious vasculopathies can primarily or secondarily involve the intracranial vasculature. Infectious vasculitides can occur in both immunocompetent and immunocompromised individuals. Vascular complications are common in the setting of pyogenic bacterial meningitis with vessel wall invasion by inflammatory cells and/or exposure to subarachnoid inflammatory exudates. Varicella zoster virus (VZV) can produce unifocal or multifocal large artery stenoses depending on the immune status of the individual. , , Although secondary CNS vasculitides are more common than PACNS, their incidence is influenced by the incidence of the underlying systemic disease.
Differentiation between PACNS and secondary CNS vasculitis is important as treatment regimens often differ. Calabrese and Mallek proposed diagnostic criteria for PACNS that include (1) an acquired or otherwise unexplainable neurologic or psychiatric condition, (2) classic features of angiitis on angiographic or histopathologic examination, and (3) no evidence of secondary vasculitis. Brain biopsy is often a necessary part of the diagnostic workup. Unfortunately, sampling error can produce false negative results, with overall limited sensitivity. IVWI has been shown to aid in directing brain biopsy to improve the chances of diagnostic tissue sampling.
Vasculitis Intracranial Vessel Wall Imaging Findings
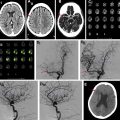
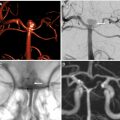
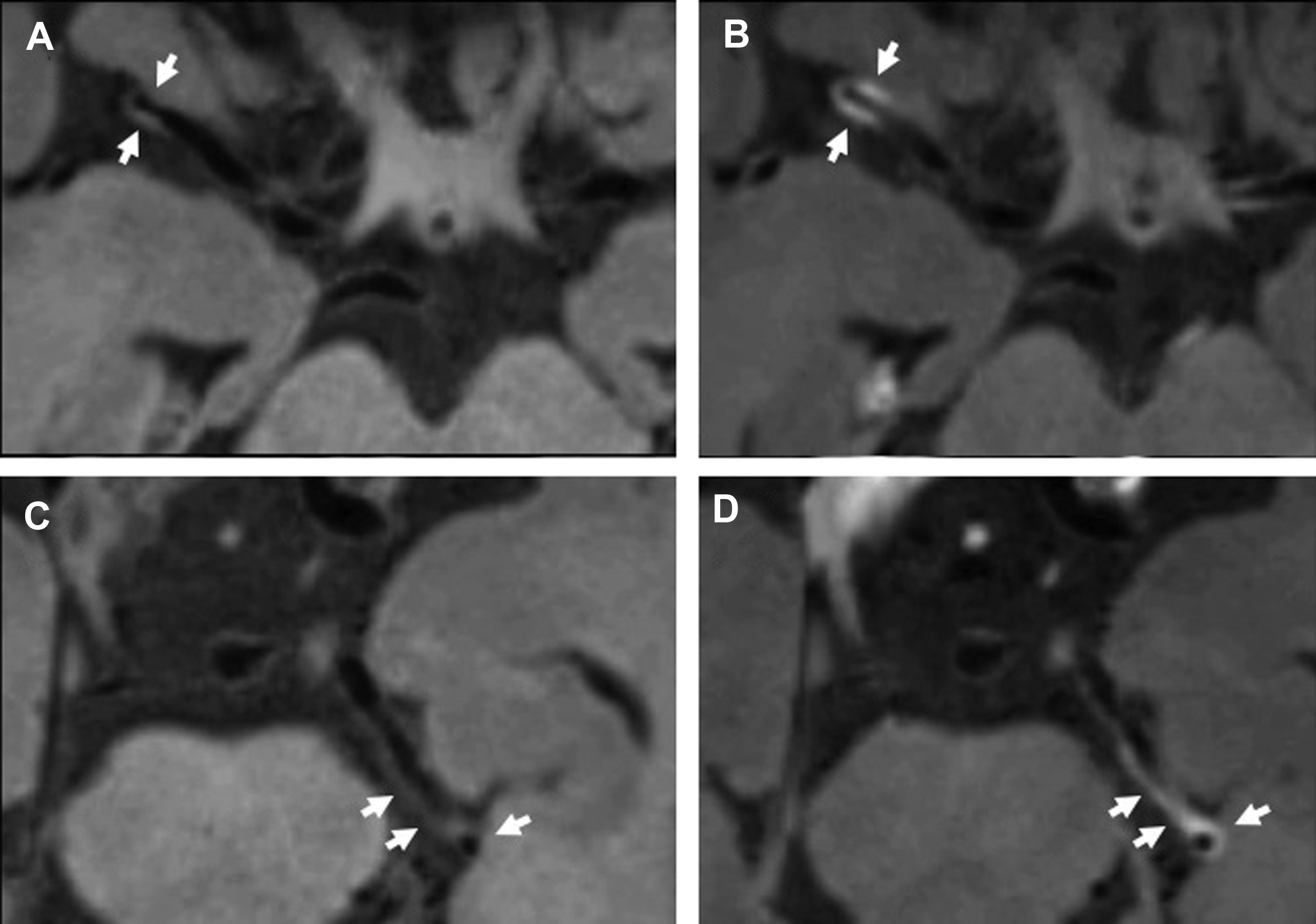
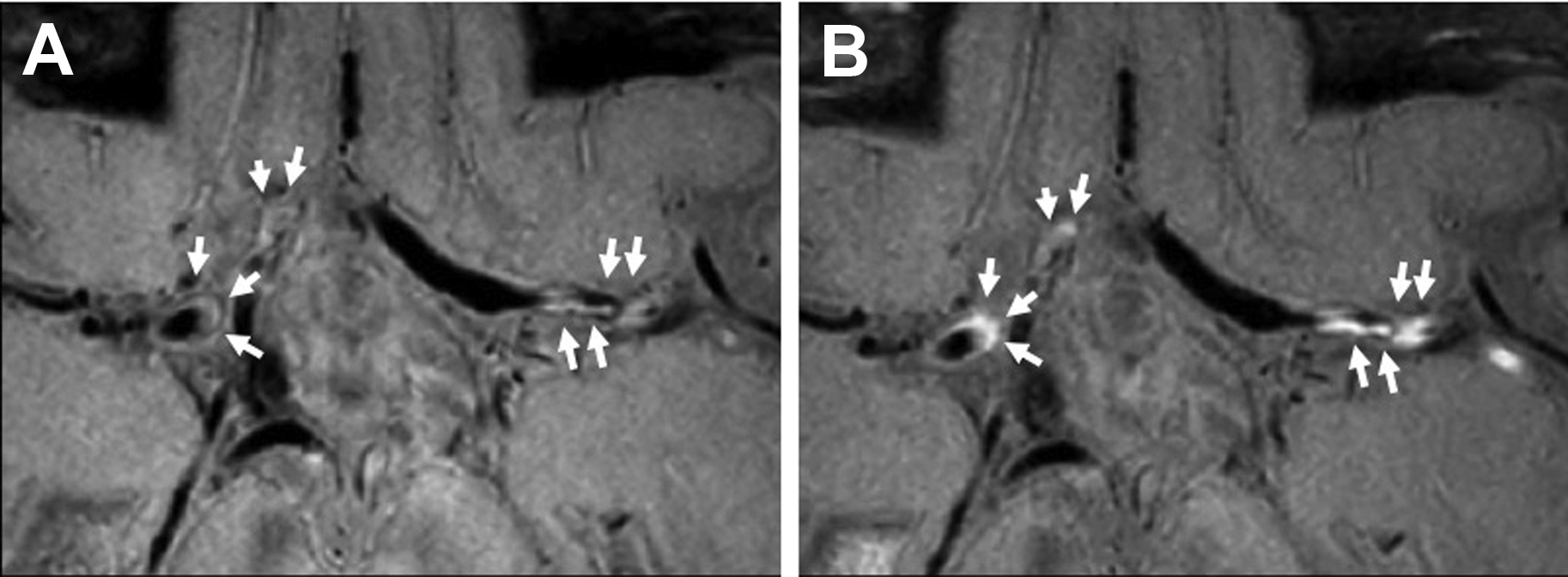
Intracranial vasculitides most commonly demonstrate concentric regions of focal wall thickening with intense enhancement ( Fig. 2 ). Wall enhancement is most commonly concentric, but eccentric enhancement can occur in a minority of cases ( Fig. 3 ). Enhancement can range from being pencil thin to thick with extension into the adjacent brain parenchyma. , , In work performed by Mossa-Basha and colleagues, all studied vasculitic lesions demonstrated diffuse enhancement with associated T2 signal abnormality that was isointense to gray matter.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree