Etiology, Prevalence, and Epidemiology
Hodgkin lymphoma (HL) is a neoplasm of B lymphocytes characterized by the presence of Reed-Sternberg cells. HL accounts for 10% of all cases of lymphoma and approximately 0.6% of all cancers diagnosed annually, with an annual incidence of 2 to 3 per 100,000 in Europe and the United States. Peak incidence occurs in two main age groups: young adults in the third decade and adults older than 50 years. It is slightly more common in men than in women, with a male to female ratio of 1.3:1 in the United States. HL is less common in Asia. Risk factors for HL include Epstein-Barr virus infection, human immunodeficiency virus infection, and positive family history.
Intrathoracic involvement in HL is common and occurs most often in the form of lymph node enlargement. Approximately 85% of patients with HL have intrathoracic disease at initial presentation. Intrathoracic involvement usually is associated with evidence of HL elsewhere in the body. In one series of 1470 patients, only 44 (3%) had purely intrathoracic disease after appropriate clinical and pathologic staging. Primary HL limited to the lung parenchyma is uncommon (<1%).
Clinical Presentation
HL typically manifests as painless lymphadenopathy, involving the cervical and/or supraclavicular regions in 60% to 80% of patients, or as an incidental mediastinal mass on chest radiograph. Systemic B symptoms, defined as unexplained and persistent fever, night sweats, and weight loss, are present in less than 20% of patients with early-stage HL and up to 50% of patients with advanced disease. Pruritus is another characteristic systemic symptom seen in 10% to 15% of patients in the early course of the disease, which initially may be mild and localized but usually progresses and becomes generalized. Localized symptoms vary with organ involvement and include chest pain, cough or shortness of breath in patients with mediastinal involvement, or dry cough in patients with pulmonary involvement.
Pathophysiology
HL is classified into two major types based on the immunophenotype of tumor cells: classic HL and nodular lymphocyte-predominant HL. Approximately 95% of cases of HL are classic HL, which is further classified histopathologically into four subtypes: nodular sclerosis (70%), mixed cellularity (10%–25%), lymphocyte-rich (5%), and lymphocyte-depleted (<1%–4%).
Of the classic HL, lymphocyte-rich and nodular sclerosis subtypes both have favorable prognosis. Nodular sclerosis HL usually occurs in young women, and most cases of HL in the mediastinum are of the nodular sclerosis subtype. The mixed-cellularity subtype tends to occur in middle-aged patients with systemic symptoms and extensive disease. The lymphocyte-depleted subtype has the worst prognosis because patients generally present with advanced-stage disease and systemic symptoms, and the bone marrow frequently is involved. Nodular lymphocyte-predominant HL is usually diagnosed before age 35 years at an early stage with indolent course, although it is often associated with late or multiple relapses. There is also associated risk of development of secondary non-Hodgkin lymphoma, particular in those with splenic involvement.
HL arises within a single lymphatic site and spreads contiguously to adjacent lymph nodes via lymphatic channels, typically spreading from the anterior mediastinal or paratracheal lymph nodes to involve, in decreasing order of frequency—hilar, subcarinal, internal mammary, pericardiophrenic, and posterior mediastinal nodes. Eventual dissemination to distant sites and organs occurs hematogenously in advanced disease. As such, pulmonary involvement is rare without mediastinal and hilar lymph node involvement.
Pathology
Histologic diagnosis of HL is made by core-needle biopsy or excisional biopsy of an involved lymph node. Typical cases of classic HL may be diagnosed on routine light microscopy based upon the presence of Reed-Sternberg giant cells in association with appropriate stromal or cellular background. The Reed-Sternberg cell is a large, bilobed cell that has prominent eosinophilic nucleoli, perinucleolar clearing, thickened nuclear membrane, and abundant cytoplasm. Histologic subtypes are differentiated based on the composition of the background infiltrate and tumor cell morphology.
Confirmatory immunophenotyping is useful for distinguishing classic HL from nodular lymphocyte-predominant HL and other types of lymphoma. Classic HL tumor cells express CD15 (85%) and CD30 (nearly 100%) and lack global expression of pan–T antigens. CD20, a B-cell antigen, and BSAP/PAX5, a regulator of B-cell development, are variably expressed. In nodular lymphocyte-predominant HL, cells are CD20 + but CD15 − and CD30 − . Reed-Sternberg cells also may express the immune checkpoint inhibitor PD-L1, which suggests potential for therapy using PD-1/PD-L1 inhibitors.
Bone marrow infiltration is seen in up to 15% of patients at diagnosis and typically occurs with advanced-stage disease. Bone marrow biopsy is no longer routinely recommended because it does not impact treatment decisions or patient outcomes, in part because of the universal use of chemotherapy and high sensitivity and specificity of positron emission tomography (PET)–computed tomography (CT) for the detection of bone marrow involvement.
Manifestations of Disease
Radiography
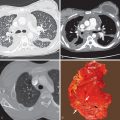
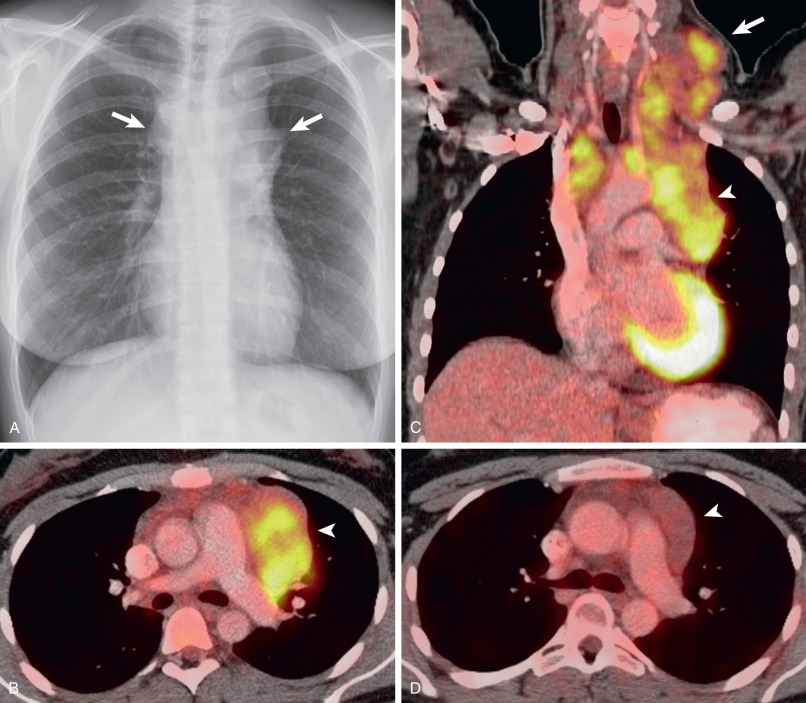
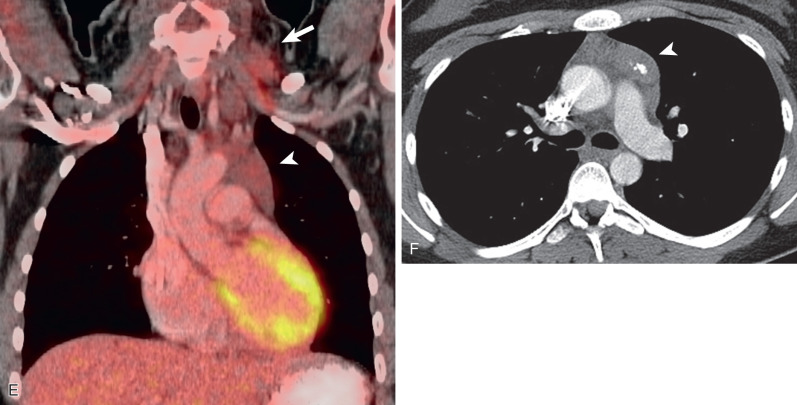
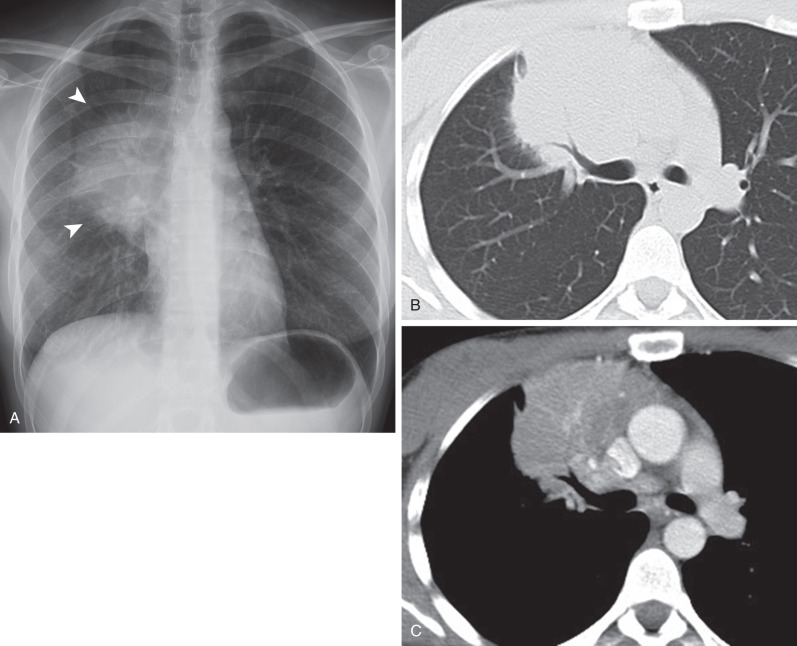
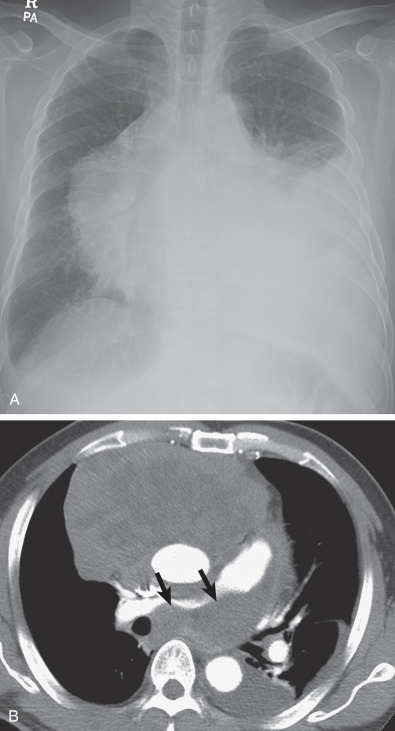
HL involving the anterior mediastinum or paratracheal lymph nodes typically manifests radiographically as widening of the superior mediastinum by a lobulated mass ( Fig. 25.1 ).
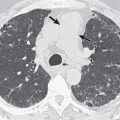
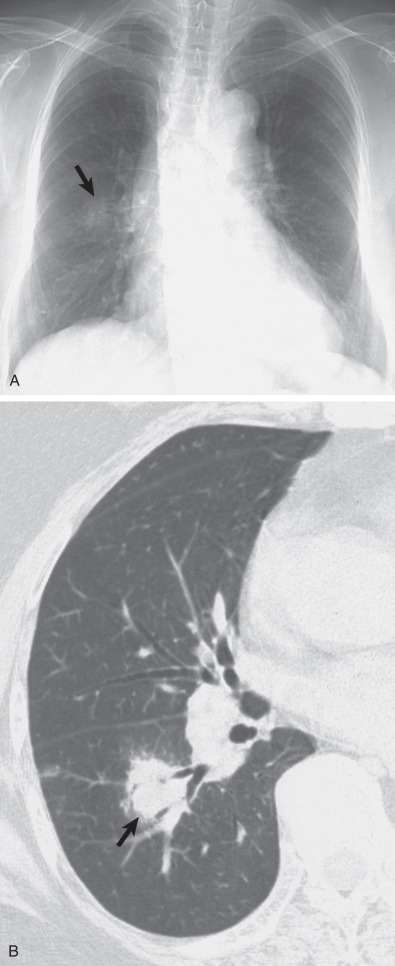
Three radiographic patterns of pulmonary parenchymal HL may occur. The most common is single or multiple pulmonary nodules, followed by lobar or segmental consolidation and reticular pattern with bronchovascular bundle and interlobular septal thickening ( Figs. 25.2 and 25.3 ). Consolidation of lung parenchyma remote from the mediastinum is common. Reticular pattern with peribronchovascular and interlobular septal thickening can result from obstruction of lymphatic or venous drainage by enlarged hilar and mediastinal lymph nodes or from intrinsic tumor deposits within interstitial lymphatic pathways. Other findings include disseminated small nodules, cavitating masses, and endobronchial nodules.
Up to 15% of patients who have HL present with bone involvement radiographically. Osseous lesions can be lytic or blastic and are usually better seen on CT.
Computed Tomography
Mediastinal Involvement
HL involves the anterior (prevascular) mediastinum or paratracheal regions in 90% to 100% of cases (see Fig. 25.1 ). In approximately 40% of cases the intrathoracic disease is confined to the anterior (prevascular) mediastinum. Enlarged lymph nodes most commonly have homogeneous soft tissue attenuation (see Fig. 25.1 ). In approximately 20% of cases, particularly in patients with large, coalescent masses, the involved lymph nodes or thymus may contain areas of low attenuation representing necrosis, hemorrhage, and cystic degeneration ( Fig. 25.4 ). Histologically, necrotic areas range from minute foci of fibrinoid necrosis to large areas of granular tissue destruction containing necrotic cells. Necrosis is seen most commonly in the nodular sclerosis and mixed-cellularity cell subtypes of HL and is not seen in the lymphocyte-rich subtype. The necrotic or cystic appearance of mediastinal lymphadenopathy in patients with HL does not affect overall survival or length of remission.
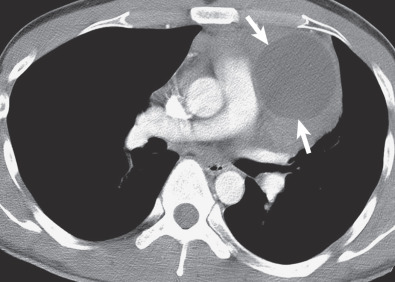
HL also frequently involves the thymus and may result in diffuse thymic enlargement. Thymic involvement may be difficult to distinguish from anterior mediastinal lymph node involvement.
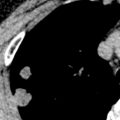
Dystrophic calcification may develop in involved lymph nodes after mediastinal radiation ( Fig. 25.5 ; see also Fig. 25.1 ). Although some investigators consider the complication to be unrelated to the degree of radiation, others link it with relatively high doses. The time interval between irradiation and the appearance of calcification ranges from 1 to 9 years.
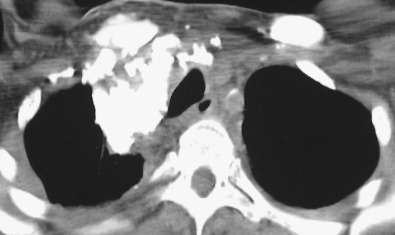
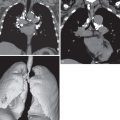
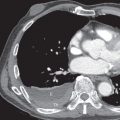
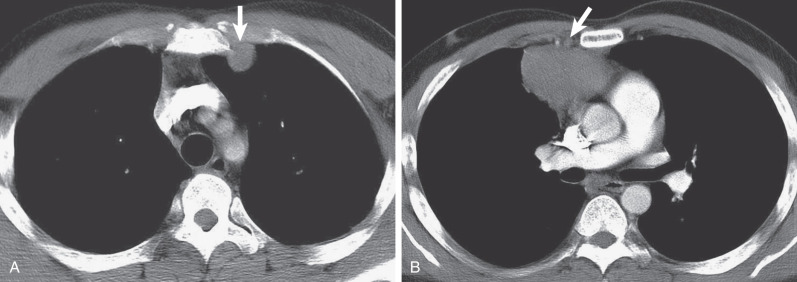
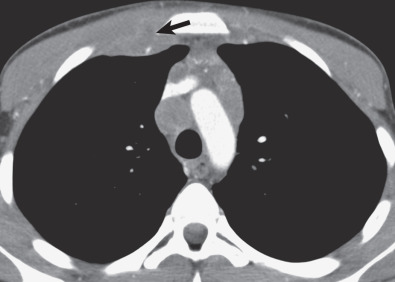
HL may extend beyond affected lymph nodes into mediastinal interstitial tissue and invade the esophagus, superior vena cava, and pericardium ( Fig. 25.6 ). Corresponding imaging manifestations, such as pericardial effusion, may be seen. In contrast to lung cancer, HL rarely invades the phrenic nerve. Involvement of the anterior mediastinal and internal mammary nodes ( Figs. 25.7 ) may be associated with invasion of the sternum or the parasternal tissues ( Fig. 25.8 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree