Hypertrophic cardiomyopathy (HCM) is a common genetic cardiomyopathy, is present in potentially as many as 1 : 200 individuals in the general population, and is caused by over 1400 mutations in at least 11 genes encoding proteins of the cardiac sarcomere. This genetic diversity is largely responsible for the heterogeneous phenotypic expression associated with this disease, including the range in the pattern of left ventricular (LV) hypertrophy ( Fig. 36.1 ) and LV outflow obstruction due to mitral valve-septal contact. Although HCM is associated with normal longevity in the vast majority of patients, a small but important subset of patients are at risk for a number of adverse clinical outcomes, including development of advanced heart failure (HF) symptoms, atrial and ventricular arrhythmias, thromboembolic events, and sudden death. Indeed, HCM remains the most common cause of sudden death in young people, including competitive athletes.

Over the last 40 years, cardiovascular imaging has had a central role in the clinical evaluation of HCM patients, with two-dimensional (2D) transthoracic echocardiography (TTE) the primary imaging technique over this period. However, over the last two decades, cardiovascular magnetic resonance (CMR) has emerged with greater frequency in clinical HCM practice as a technique well-suited to characterize the HCM phenotype with noninvasive, nonionizing radiation, high spatial resolution imaging, and complete tomographic reconstruction of the entire cardiac chamber (without obliquity), unencumbered by a limited acoustic window or interference from thoracic and pulmonary parenchyma. In addition, contrast-enhanced CMR with late gadolinium enhancement (LGE) has the capability of identifying areas of focal fibrosis/scarring, information not obtainable with echocardiography. In this chapter, we discuss areas in which CMR has contributed to the routine clinical evaluation of HCM patients.
Diagnosis
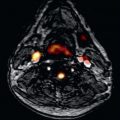
A diagnosis of HCM is made when unexplained LV hypertrophy (≥15 mm wall thickness; see Fig. 36.1 ) occurs in the absence of another disease capable of producing the magnitude of increased wall thickness. CMR, by virtue of its high spatial resolution and sharp contrast between bright-blood and dark myocardium, allows for precise and reliable noninvasive measurements of LV wall thickness and can clarify the diagnosis when wall thickness measurements fall in the nondiagnostic range (i.e., normal or borderline increased) by TTE ( Fig. 36.2 ). In particular, focal areas of increased wall thickness may not be well seen by TTE (“echo blind”) when confined to three specific regions of the LV chamber, including anterolateral wall, apex, and inferior septum ( Fig. 36.3 ). For these reasons, when a diagnosis of HCM is suspected based on clinical profile and TTE is normal/nondiagnostic, CMR should be routinely performed to clarify the diagnosis (see Fig. 36.2 ).


In addition, the extent of LV wall thickness may similarly be underestimated by TTE, with more accurate measurements made by CMR. Discrepancy in wall thickness measurements between the two techniques is often a result of underestimation of segmental areas of LV hypertrophy present in the aforementioned “echo blind” regions of the LV wall (see Fig. 36.3 ). This observation can have direct management implications, because massive hypertrophy (wall thickness ≥30 mm) is an independent risk factor for sudden death in HCM, and in some patients these extents of hypertrophy may only be recognized by CMR.
In addition, overestimation of wall thickness can also occur if the crista supraventricularis, a prominent right ventricular (RV) muscle structure that originates in the RV apex and transects the cavity to inset adjacent to the ventricular septum, is included in the transdimensional measurement of ventricular basal septal thickness ( Fig. 36.4B ). With CMR, the crista can be reliably identified on the basal short-axis images and observed to separate from the septum in diastole, exposing the epicardial border of the interventricular septum and allowing for the epicardial border of ventricular septum to be clearly delineated.

Phenotype Characterization of Hypertrophic Cardiomyopathy
Patterns and Distribution of Left Ventricular Hypertrophy
The most common location of focal LV wall thickening in HCM involves the basal anterior free wall in continuity with the anterior ventricular septum (see Fig. 36.1 ). This phenotype is present in three-quarters of HCM patients. The inferior septum at the mid-LV level is the next most common region of increased wall thickness. A small subset (10%) of HCM patients have LV hypertrophy, which is focal and limited involving only one or two LV segments, including the apex and the majority of these patients will have normal LV mass by CMR. These observations underscores the principle that a diagnosis of HCM is compatible with limited extent of wall thickening and that increased LV mass is not a requirement for the clinical diagnosis of HCM. Of note, a subgroup of HCM patients demonstrate a noncontiguous distribution of LV hypertrophy, a pattern in which hypertrophied segments are separated (often abruptly) by segments of normal wall thickness.
Right Ventricle
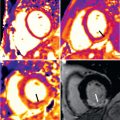
A number of CMR observations have demonstrated the presence of morphologic RV abnormalities in HCM. A significant proportion of HCM patients demonstrate increased RV wall thickness by CMR (i.e., ≥8 mm), which is well suited to reliably visualize morphologic abnormalities of the RV. Areas of increased RV wall thickness are most commonly seen near the junction of the insertion of the RV wall into either the anterior or inferior septum; however, diffuse RV hypertrophy can also occur ( Fig. 36.4A ). Although uncommon, RV LGE can also occur in HCM. Currently, the clinical significance of RV hypertrophy or LGE, is uncertain.
Left Ventricular Apical Aneurysm
Increasing penetration of CMR into routine cardiovascular practice has resulted in more frequent identification of a subset of patients with thin-walled, scarred LV apical aneurysms ( Fig. 36.4C ) often associated with muscular mid-cavity obstruction. This important group of patients had been underdiagnosed before the application of CMR to HCM, because small-to-moderately sized aneurysms may not be reliably identified by TTE. LGE CMR has demonstrated that the aneurysm rim in these patients is composed predominantly of fibrosis that extends from the aneurysm rim into the septum and free wall and represents a primary arrhythmogenic substrate for the generation of malignant ventricular tachyarrhythmias.
This subgroup of HCM patients is considered high risk, with adverse event rates of 6% per year (over 3-fold greater than that of HCM cohorts without aneurysms), a result largely of sudden death events and thromboembolic stroke (secondary to LV thrombus formation in the aneurysmal cavity). Therefore identification of this unique phenotype raises important management considerations because these complications are effectively treatable with primary prevention implantable cardioverter defibrillator (ICD) therapy, radiofrequency ablation for recurrent ventricular tachycardia (VT), and prophylactic anticoagulation for stroke prevention.
Assessment of Hypertrophic Cardiomyopathy Family Members
HCM is a genetic disorder; therefore screening of all first-degree relatives of HCM patients is indicated to identify those individuals with potentially unrecognized disease. Screening should begin at the onset of adolescence, with repeat imaging performed annually (every 12 to 18 months) throughout adolescence, and then every 5 years until the fourth decade of life, because delayed-onset hypertrophy can occur in adulthood in a small number of patients. Although TTE has been the mainstay test used for longitudinal family screening, CMR has been recognized to have an increasing role because it provides a more precise delineation of LV hypertrophy and therefore more accurate diagnosis. In addition, CMR provides a benchmark for future studies to better define the potential progression of LV hypertrophy.
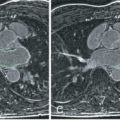
In addition, CMR can assess for a variety of morphologic abnormalities that may be present in HCM family members who carry a disease-causing sarcomere mutation but without LV hypertrophy (i.e., genotype positive–phenotype negative; G+P−) patients, including myocardial crypts (i.e., narrow deep blood-filled invaginations within LV myocardium; Fig. 36.4F ), elongated mitral valve leaflets, expanded extracellular space (with T1 mapping), and LGE. These observations suggest that even in the absence of LV hypertrophy, the hearts of patients who are G+P- may be morphologically abnormal. Finally, the presence of one or more of these structural abnormalities identified in a family member of an HCM patient should prompt continued close surveillance with serial CMR for development of LV hypertrophy and conversion to clinical disease and/or genetic testing to aid in confirming HCM diagnosis.
Differentiation of Other Etiologies of Left Ventricular Hypertrophy
Athletic Heart
LV hypertrophy associated with systemic training (i.e., athletic heart) may be difficult to differentiate from HCM. CMR can help distinguish by identification of focal pattern of hypertrophy, a finding supportive of a diagnosis of HCM. In addition, forced deconditioning of an athlete may serve as a useful strategy to resolve diagnosis, with CMR well suited to compare maximum LV wall thickness measurements before and after a period of systemic deconditioning. In this regard, a patient whose wall thickness regresses more than 2 mm supports a diagnosis of athletic heart, whereas LV hypertrophy that remains present despite deconditioning supports a diagnosis of HCM. Myocardial tagging using spatial modulation of magnetization (SPAMM) may also be useful in discriminating physiologic from pathologic hypertrophy.
LGE CMR provides the opportunity to aid in the differentiation given the ability to noninvasively provide tissue characterization by means of identifying focal areas of replacement fibrosis and expanded extracellular space. Although LGE is present in about half of individuals with HCM, LV remodeling associated with the athlete heart should not result in focal areas of myocardial scarring/fibrosis, especially in younger individuals. Therefore in an athlete in whom suspicion has been raised for HCM, the presence of LGE on contrast-enhanced CMR favors a diagnosis of HCM. In contrast, the absence of LGE does not exclude HCM because only half of HCM patients demonstrate LGE.
Hypertensive Cardiomyopathy
The differentiation of LV hypertrophy because of systemic hypertension from HCM has historically been challenging. CMR can help in differentiation by examining the pattern of hypertrophy. With long-standing systemic hypertension, concentric hypertrophy (near-identical hypertrophy in septum and lateral wall) is predominant, whereas LV wall thickening in HCM is almost always asymmetric. Additionally, an LV outflow obstruction because of typical systolic anterior motion of the mitral valve will also sway a diagnosis toward HCM. This finding is present in over one-third of HCM patients and very rarely seen in hypertensive cardiomyopathy. CMR can also be helpful in the detection of changes in serial measurements of LV wall thickness after aggressive treatment with antihypertensives, in which a regression of hypertrophy would favor a diagnosis of hypertensive cardiomyopathy.
Left Ventricular Noncompaction
Because of its superior spatial resolution with imaging of the LV apex, CMR may also help to clarify (or alter) diagnosis by demonstrating the presence of prominent trabeculations consistent with a diagnosis of left ventricular noncompaction (LVNC) in patients initially diagnosed with apical HCM. LV trabeculations, associated with LVNC, when imaged by 2D TTE, may appear as apical hypertrophy, potentially resulting in a misdiagnosis of apical HCM. This has important implications for management strategies because a diagnosis of LVNC has significantly different treatment implications (including participation in competitive sports, anticoagulation, and risk stratification for sudden cardiac death).
Infiltrative Cardiomyopathy
Infiltrative cardiomyopathies, including amyloidosis or glycogen/lysosomal storage diseases (such as Anderson-Fabry disease or Danon disease) can mimic HCM because they can produce increased wall thickness as part of their phenotypic expression. Although these diseases may have noncardiac signs and symptoms, disease expression can be confined only to the heart. The accurate differentiation of these “photocopies” is critical because treatment strategies and prognosis differs compared with HCM. In amyloidosis, CMR identification of nearly identical wall thickness measurements in the septum and lateral wall (“concentric”) combined with global subendocardial LGE is suggestive for cardiac amyloidosis and is not typical in HCM ( Fig. 36.5 ). Marked hypertrophy in the absence of electrocardiographic (ECG) evidence for LV hypertrophy should also raise the clinician’s suspicion for an infiltrative/amyloid cardiomyopathy. Suspicion for Anderson-Fabry disease, an X-linked storage disease in which mutations in the α-galactosidase A gene lead to cellular accumulation of glycosphingolipids in multiple organs including the heart, is potentially treatable with enzyme replacement therapy and can be raised by a concentric pattern of hypertrophy by CMR with LGE confined to the basal inferolateral wall (see Fig. 36.5 ).